- Department of Neurological Surgery, University of Alabama at Birmingham, Birmingham, AL, USA
- Department of Medicine, Division of Infectious Diseases, University of Alabama at Birmingham, Birmingham, AL, USA
- Department of Pathology, Division of Neuropathology, University of Alabama at Birmingham, Birmingham, AL, USA
Correspondence Address:
Samuel G. McClugage
Department of Neurological Surgery, University of Alabama at Birmingham, Birmingham, AL, USA
DOI:10.4103/sni.sni_157_17
Copyright: © 2017 Surgical Neurology International This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Samuel G. McClugage, Rachael A. Lee, Bernard C. Camins, Juan J. Mercado-Acosta, Martin Rodriguez, Kristen O. Riley. Treatment of racemose neurocysticercosis. 01-Aug-2017;8:168
How to cite this URL: Samuel G. McClugage, Rachael A. Lee, Bernard C. Camins, Juan J. Mercado-Acosta, Martin Rodriguez, Kristen O. Riley. Treatment of racemose neurocysticercosis. 01-Aug-2017;8:168. Available from: http://surgicalneurologyint.com/?post_type=surgicalint_articles&p=8518
Abstract
Background:Neurocysticercosis (NCC) is a common parasitic infection of the central nervous system, caused by the tapeworm Taenia solium. It is endemic to certain parts of the world, including Central America, South America, Asia, and Africa. The racemose form, characterized by extraparenchymal location, increased morbidity and mortality, and large loculated cystic lesions, is rarely seen in industrialized countries, such as the United States. The management of racemose neurocysticercosis (RNCC) differs from that of the typical parenchymal variant. The ideal course of treatment is debated by experts, but typically includes either surgical intervention with subsequent medical therapy or medical therapy alone.
Case Description:We present the case of a 34-year-old male diagnosed with RNCC and treated successfully with surgical cyst drainage, resection, and subsequent medical therapy.
Conclusion:Currently, no standardized evidence-based protocol exists that dictate appropriate treatment for extraparenchymal or racemose NCC. We present a case of RNCC treated successfully with surgical and medical intervention. Further research encompassing well-designed clinical trials is necessary to delineate appropriate and standardized protocols for treatment of this disease.
Keywords: Cyst rupture, medication, neurocysticercosis, racemose, surgery, Taenia solium, treatment
INTRODUCTION
The pork tapeworm Taenia solium can cause two different infections in humans: intestinal taeniasis and cysticercosis.[
Central nervous system involvement, known as neurocysticercosis (NCC), occurs frequently in patients with cysticercosis, and is a common cause of new-onset seizures in endemic regions.[
Intraparenchymal NCC is the most common form and is most frequently associated with epilepsy.[
Treatment for parenchymal NCC typically consists of anti-parasitic drugs for viable brain cysts; however, appropriate therapy for RNCC is debated. Some experts have suggested open or endoscopic surgery when possible, with the goal of cyst removal, combined with oral anti-parasitic treatment.[
CASE REPORT
History/Pertinent findings
A 34-year-old right-handed Hispanic male presented initially to an another hospital after a new-onset generalized seizure while driving. He emigrated from Mexico 15 years prior to presentation and has not returned for 6 years. He traveled to many states around the US for work, including Texas, Louisiana, Virginia, Florida, and Nebraska, but has lived in Alabama for the last 11 years. On admission to an outside hospital, he underwent CT head and subsequent MRI brain, which revealed a multi-lobulated cystic lesion of the left fronto-temporal region. A lumbar puncture was performed with cerebral spinal fluid (CSF) labs, culture, cytology, and flow cytometry; all of which were unremarkable. He was evaluated by an outside neurologist and started on oral phenytoin for seizure control. He was subsequently discharged with follow-up at our facility for further management.
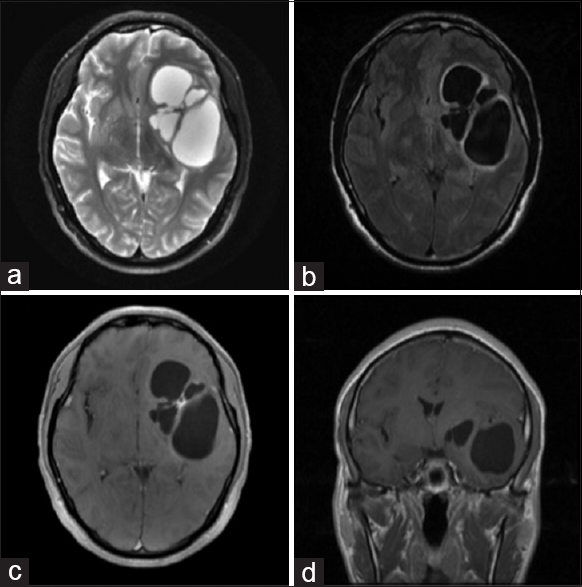
Upon evaluation in clinic, he had no focal deficits on neurological examination. Imaging from the another hospital was reviewed. The CT head non-contrast showed a large left fronto-temporal multi-lobulated cystic lesion ~6.5 cm × 4.5 cm × 4 cm in size and primarily centered around the sylvian fissure, causing significant mass effect and 0.5 cm midline shift. Calcifications were present in the central confluence of the cysts. The largest cyst cavity was in the left temporal lobe and did not appear to communicate with the temporal horn. The MRI brain, with and without contrast with T2-weighted sequences, revealed high intensity within the cyst, similar to CSF [
Figure 1
Pre-operative MRI brain with and without contrast was obtained prior to intervention, showing a large left multi-lobulated cystic lesion, causing significant mass effect and midline shift. The cyst contents appears isointense to CSF on MRI imaging. No evidence of enhancement was evident on contrasted imaging. Sequences are as follows: (a) Axial T2 sequence, (b) Axial FLAIR, (c) Axial with contrast, and (d) Coronal with contrast
Surgical treatment
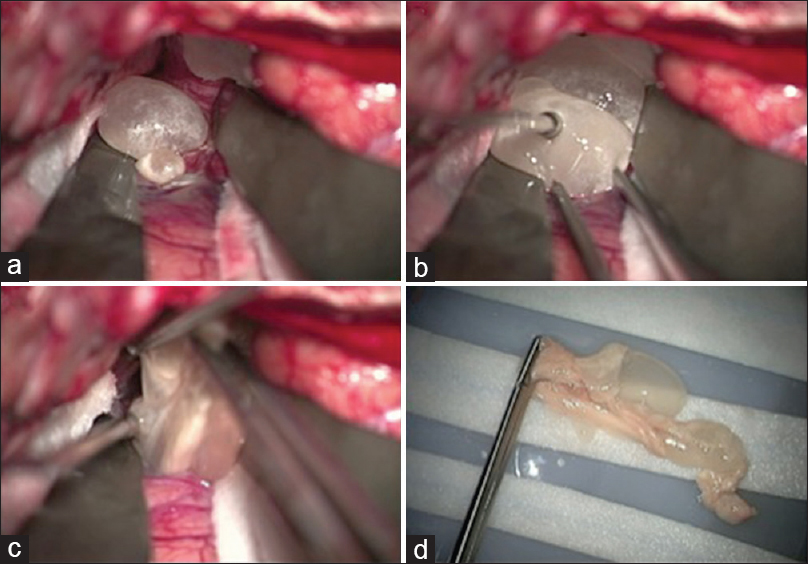
The patient was treated with dexamethasone 8 mg intravenously every 8 hours on the day prior to surgery and his home phenytoin dose was continued. The CT of his chest, abdomen, and pelvis with contrast were obtained to rule out other possible diagnoses on the differential, including metastatic disease and hydatid cysts; this was unremarkable. The patient was taken to the operating room the following day for surgery and pre-treated with 10 mg of dexamethasone intravenously. A large left pterional craniotomy was performed to sufficiently expose portions of the left frontal and temporal lobes, as well as the sylvian fissure (see
Figure 2
Left craniotomy and sylvian fissure dissection is shown. (a) Evagination of the cyst wall with initial sylvian dissection. (b) Gentle traction is applied to the cyst to aid in removal. (c) The cyst ruptures during the process of removal. Copious irrigation was performed to clear cyst contents and the patient was given another dose of dexamethasone. (d) Gross specimen of multi-cystic racemose neurocysticercosis after en bloc resection
Postoperative course
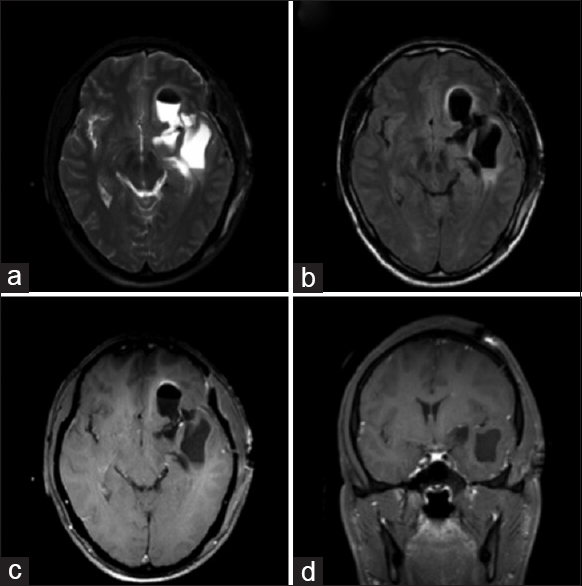
Post-operatively, the patient was started on a 14-day course of oral albendazole 400 mg twice daily. He was discharged from the hospital on post-operative day 5 without complications. He was treated with dexamethasone while undergoing oral anti-parasitic therapy and discharged on a slow steroid taper. He remained on his admission dose of phenytoin at discharge. On scheduled outpatient follow-up, he felt well. Surveillance CT head revealed stable left fronto-temporal encephalomalacia and no evidence of recurrence [
Pathological findings
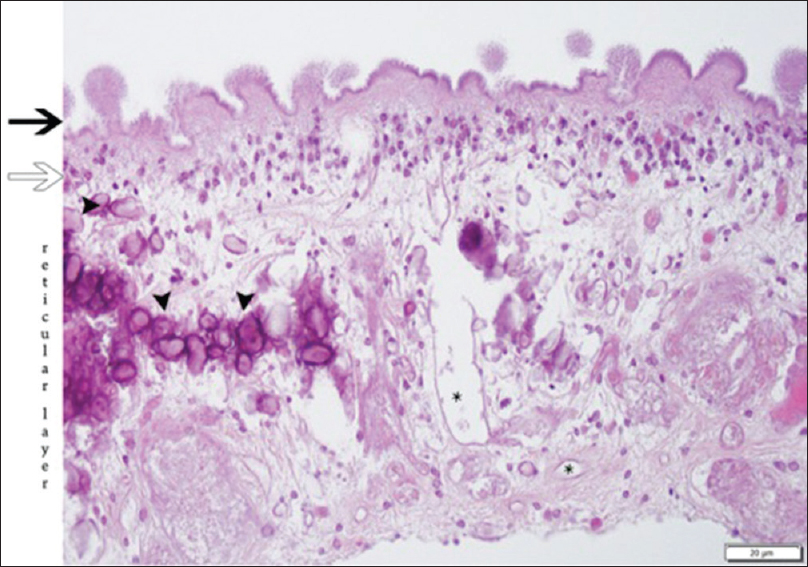
Neuropathology was consistent with neurocysticercosis in racemose cyst formation [
Figure 5
The cuticular layer (black arrow) shows an outer portion with a convoluted appearance of rounded protrusions (“knobs”) and microtriches on the surface. The cellular layer (white arrow) is comprised of a syncytium of cell bodies with uniform nuclei. The inner reticular layer contains loosely arranged fibrils, calcifications (arrowheads), as well as excretory canaliculi (asterisks). H & E stain, 400X magnification
DISCUSSION
There is abundant medical literature on the treatment of NCC in general, however, the racemose form of NCC is less commonly encountered, thus limited literature and no evidence-based guidelines exist discussing the appropriate treatment of this enigmatic and deadly disease.[
Diagnosis
Diagnosis of extraparenchymal neurocysticercosis can be difficult, given that histological confirmation is often not possible without surgical intervention, and is typically based on imaging and serologic findings.[
Serological studies detect antibodies to the T. solium within serum or CSF. The best serological test for NCC is the enzyme-linked immunoelectrotransfer blot (EITB) assay to detect serum antibodies, with sensitivity reported around 98% for patients with two or more live parasites.[
Medical therapy
While guidelines exist regarding the management of NCC in general, there is no consensus on the management of RNCC regarding both surgical and medical means.[
Surgical therapy
Surgical therapy for RNCC remains controversial. Some authors advocate for surgical intervention for resection, either through open surgical or endoscopic approaches, dependent on cyst location.[
Intra-operative cyst rupture
One of the possible risks of operative intervention for RNCC is the possibility of intra-operative cyst rupture. This can cause an acute and often fatal inflammatory response, which given the subarachnoid location of the racemose form, puts the patient at risk for vasospasm and stroke.[
In our patient, we chose open surgical removal given the low chance of success with medical therapy alone, the accessible location of the lesion, and extreme amount of mass effect and midline shift caused by it. Dexamethasone therapy was initiated given evidence of peri-lesional edema on MRI and associated mass effect. Treatment with pre and postoperative steroids, as well as intra-operative irrigation proved sufficient in avoiding the inflammatory sequelae of cyst rupture. It is likely that rupture was unavoidable in this particular case and may have simply been due to the initial decompression of the cyst with a Touhey needle, which proved necessary to provide the needed decompression to safely attempt sylvian dissection. A generous craniotomy to expose the affected area also proved to be essential in safe resection. Albendazole mono-therapy was chosen, as it is a common treatment for NCC, although recent studies have shown efficacy with albendazole and praziquantel in combination, and may be also considered as an option.[
CONCLUSIONS
Racemose NCC remains a less common form of NCC and is an especially difficult disease to treat. No consensus exists on the proper treatment protocols for this particular variety of NCC. Further study is needed to define a consensus standard of care for patients with this entity. We present a case of RNCC in a non-endemic region treated successfully with both open surgical and medical therapy, as well as management of intra-operative cyst rupture.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Video Available on: www.surgicalneurologyint.com
References
1. Agapejev S. Neurocysticercosis: The enigmatic disease. Cent Nerv Syst Agents Med Chem. 2011. 11: 261-84
2. Anqi X, Jiahe X, Xiaoke Z, Chao Y. The surgical value of Neurocysticercosis: Analyzing 10 patients in 5 years. Turk Neurosurg. 2015. p.
3. Baird RA, Wiebe S, Zunt JR, Halperin JJ, Gronseth G, Roos KL. Evidence-based guideline: Treatment of parenchymal neurocysticercosis: Report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology. 2013. 80: 1424-9
4. Bansal R, Gupta M, Bharat V, Sood N, Agarwal M. Racemose variant of neurocysticercosis: A case report. J Parasit Dis. 2016. 40: 546-9
5. Carpio A, Fleury A, Romo ML, Abraham R, Fandiño J, Durán JC. New diagnostic criteria for neurocysticercosis: Reliability and validity. Ann Neurol. 2016. 80: 434-42
6. Carrillo Mezo R, Lara García J, Arroyo M, Fleury A. Relevance of 3D magnetic resonance imaging sequences in diagnosing basal subarachnoid neurocysticercosis. Acta Trop. 2015. 152: 60-5
7. Colli BO, Carlotti CG, Assirati JA, Machado HR, Valença M, Amato MCM. Surgical treatment of cerebral cysticercosis: Long-term results and prognostic factors. Neurosurg Focus. 2002. 12: E3-
8. Couldwell WT, Zee CS, Apuzzo ML. Definition of the role of contemporary surgical management in cisternal and parenchymatous cysticercosis cerebri. Neurosurgery. 1991. 28: 231-7
9. Fleury A, Carrillo-Mezo R, Flisser A, Sciutto E, Corona T. Subarachnoid basal neurocysticercosis: A focus on the most severe form of the disease. Expert Rev Anti Infect Ther. 2011. 9: 123-33
10. Garcia HH, Evans CAW, Nash TE, Takayanagui OM, White AC, Botero D. Current consensus guidelines for treatment of neurocysticercosis. Clin Microbiol Rev. 2002. 15: 747-56
11. Garcia HH, Lescano AG, Gonzales I, Bustos JA, Pretell EJ, Horton J. Cysticidal Efficacy of Combined Treatment With Praziquantel and Albendazole for Parenchymal Brain Cysticercosis. Clin Infect Dis. 2016. 62: 1375-9
12. Garcia HH, Nash TE, Del Brutto OH. Clinical symptoms, diagnosis, and treatment of neurocysticercosis. Lancet Neurol. 2014. 13: 1202-15
13. Göngora-Rivera F, Soto-Hernández JL, González Esquivel D, Cook HJ, Márquez-Caraveo C, Hernández Dávila R. Albendazole trial at 15 or 30 mg/kg/day for subarachnoid and intraventricular cysticercosis. Neurology. 2006. 66: 436-8
14. Gonzales I, Rivera JT, Garcia HH. Pathogenesis of Taenia solium taeniasis and cysticercosis. Parasite Immunol. 2016. 38: 136-46
15. Hawk MW, Shahlaie K, Kim KD, Theis JH. Neurocysticercosis: A review. Surg Neurol. 2005. 63: 123-32
16. Jiménez-Vázquez OH, Nagore N. Endoscopic evidence of ventricular and cisternal inflammatory changes after intraoperative cysticercal rupture during endoscopic third-ventriculostomy removal. Br J Neurosurg. 2013. 27: 137-8
17. Krupa K, Krupa K, Pisculli ML, Athas DM, Farrell CJ. Racemose neurocysticercosis. Surg Neurol Int. 2016. 7: 12-
18. Lerner A, Shiroishi MS, Zee CS, Law M, Go JL. Imaging of neurocysticercosis. Neuroimaging Clin N Am. 2012. 22: 659-76
19. Machado DC, Camilo GB, Alves UD, de Oliveira CE, de Oliveira RV, Lopes AJ. Imaging aspects of the racemose neurocysticercosis. Arch Med Sci AMS. 2015. 11: 1356-60
20. Mahale RR, Mehta A, Rangasetty S. Extraparenchymal (Racemose) Neurocysticercosis and Its Multitude Manifestations: A Comprehensive Review. J Clin Neurol (Seoul, Korea). 2015. 11: 203-11
21. Michelet L, Fleury A, Sciutto E, Kendjo E, Fragoso G, Paris L. Human neurocysticercosis: Comparison of different diagnostic tests using cerebrospinal fluid. J Clin Microbiol. 2011. 49: 195-200
22. Proaño JV, Madrazo I, Avelar F, López-Félix B, Díaz G, Grijalva I. Medical treatment for neurocysticercosis characterized by giant subarachnoid cysts. N Engl J Med. 2001. 345: 879-85
23. Rajshekhar V. Surgical management of neurocysticercosis. Int J Surg (Lond Engl). 2010. 8: 100-104
24. Rangel-Castilla L, Serpa JA, Gopinath SP, Graviss EA, Diaz-Marchan P, White AC. Contemporary neurosurgical approaches to neurocysticercosis. Am J Trop Med Hyg. 2009. 80: 373-8
25. Sharma S, Modi M, Lal V, Prabhakar S, Bhardwaj A, Sehgal R. Reversible dementia as a presenting manifestation of racemose neurocysticercosis. Ann Indian Acad Neurol. 2013. 16: 88-90
26. Sinha S, Sharma BS. Neurocysticercosis: A review of current status and management. J Clin Neurosci. 2009. 16: 867-76
27. Takayanagui OM, Odashima NS. Clinical aspects of neurocysticercosis. Parasitol Int. 2006. 55: S111-5
28. Tuzun Y, Kadioglu HH, Izci Y, Suma S, Keles M, Aydin IH. The clinical, radiological and surgical aspects of cerebral hydatid cysts in children. Pediatr Neurosurg. 2004. 40: 155-60
29. Umredkar A, Singla N, Mohindra S, Bal A, Gupta SK. Giant intraparenchymal neurocysticercosis: Report of surgical aspects two cases. Neurol India. 2009. 57: 800-2