- Department of Neurosurgery, Inselspital, Bern University Hospital, Freiburgstrasse, Bern, Switzerland
- Department of Radiology, Spital Männedorf, Asylstrasse, Männedorf, Switzerland
Correspondence Address:
Philippe Schucht
Department of Neurosurgery, Inselspital, Bern University Hospital, Freiburgstrasse, Bern, Switzerland
DOI:10.4103/sni.sni_313_17
Copyright: © 2017 Surgical Neurology International This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Andrea Bodmer, Steffen Ross, Andreas Raabe, Jürgen Beck, Christian T. Ulrich, Philippe Schucht. Virtual autopsy to assess sacral anatomy: Conditions for a minimal invasive approach to the spinal canal through the hiatus sacralis. 06-Dec-2017;8:290
How to cite this URL: Andrea Bodmer, Steffen Ross, Andreas Raabe, Jürgen Beck, Christian T. Ulrich, Philippe Schucht. Virtual autopsy to assess sacral anatomy: Conditions for a minimal invasive approach to the spinal canal through the hiatus sacralis. 06-Dec-2017;8:290. Available from: http://surgicalneurologyint.com/?post_type=surgicalint_articles&p=8691
Abstract
Background:Despite multiple advantages of minimally invasive techniques in spinal surgery, the currently used approaches may lead to postoperative pain and spinal instability. As a natural orifice, the hiatus sacralis offers a nontransmuscular alternative entry point for endoscopic approaches. In this study, we collected data about the complex anatomical conditions of the sacral canal as a basis for the development of a sacral endoscope.
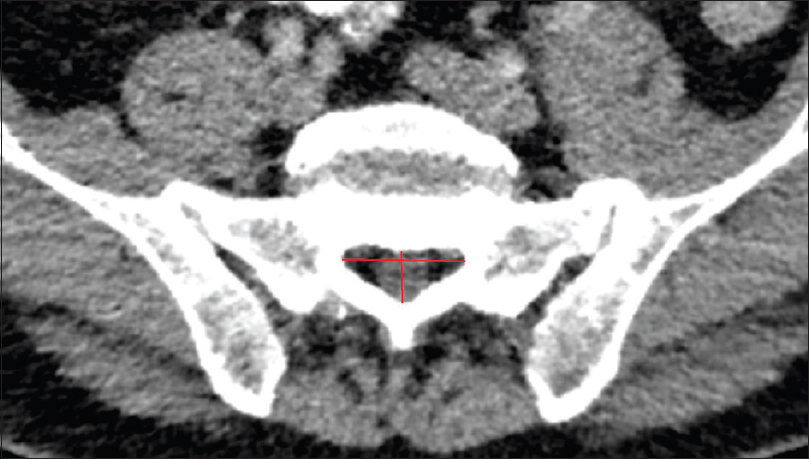
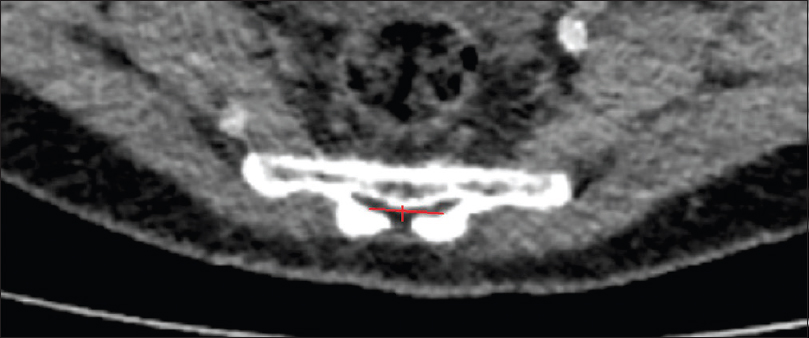
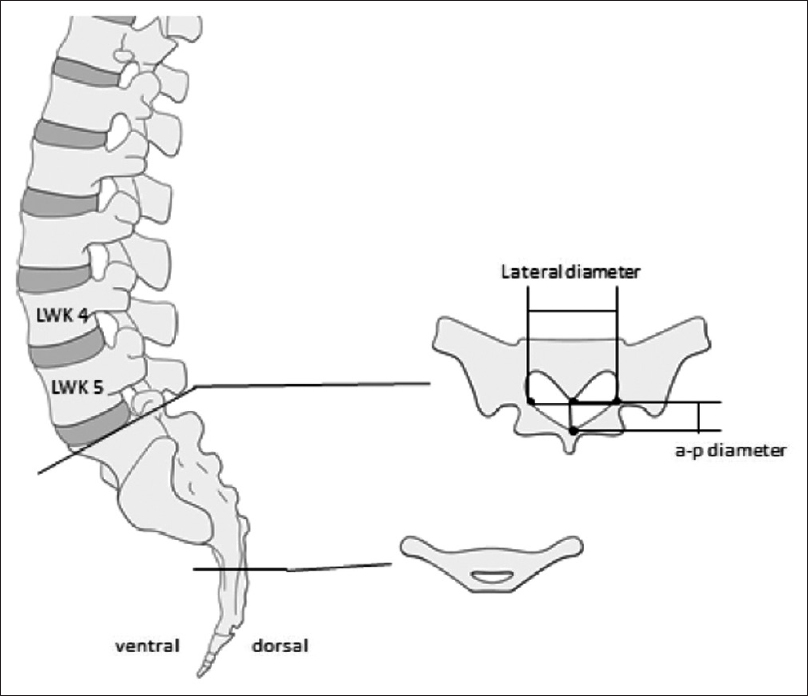
Methods:We retrospectively evaluated 192 postmortem human cadaveric specimens with computed tomography (CT). The anatomical conditions of the sacrum and lumbar spine were analyzed, including assessment of the lateral and anteroposterior diameters, measurement of the cross-sectional area of the sacral canal at the lumbosacral transition, hiatus sacralis, and the narrowest point of the sacral canal.
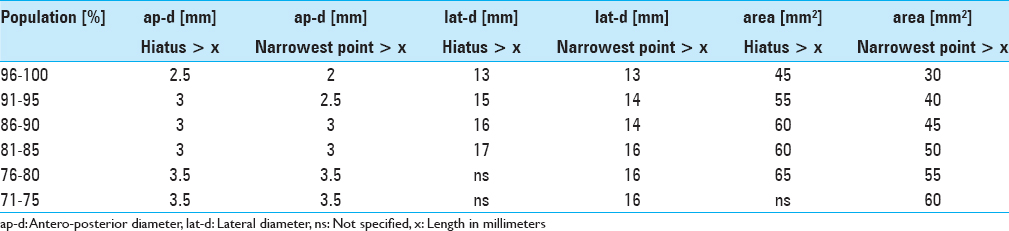
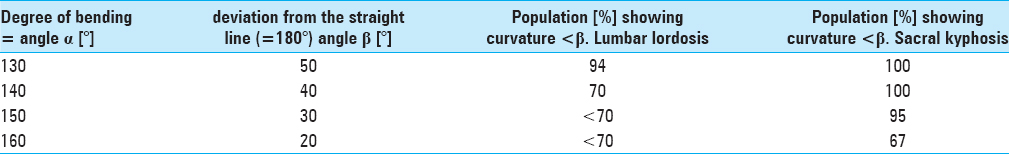
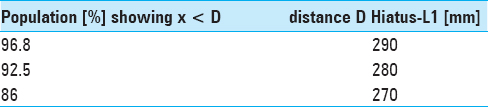
Results:The narrowest anteroposterior diameter was >2.3 mm in 95% of the cases; the width was >13 mm in 95% of the cases. The narrowest point was located at the hiatus in 72% of the cases. The angle of sacral kyphosis was less than 30° and less than 50° in lumbar lordosis in 95% of the cases. A length shorter than 288 mm was measured in 95% of the cases. Anatomical conditions in male and female sacra were comparable.
Conclusions:The narrow anteroposterior diameter is the key limiting feature for using the canalis sacralis as a natural entry point into the spinal canal. Sacroscopy will require endoscopes with a flattened shape, with parallel arrangement of instruments and flexibility to accommodate the varied dorsal and ventral curvatures.
Keywords: Chronic lumbar pain, endoscopy, minimally invasive surgery, sacral anatomy, sacroscopy, spinal surgery
INTRODUCTION
Epiduroscopic spinal surgery as a minimally invasive procedure was first reported in the 1930s when Burman used arthroscopic equipment for the assessment of spinal pathology.[
In theory, minimally invasive (MIS) endoscopic techniques offer shorter operating times, short rehabilitation period, and reduced tissue trauma compared with routine open approaches.[
MATERIALS AND METHODS
Sacral and lumbar spinal anatomical measurements were based on computed tomography (CT) data sets from the Institute of Forensic Medicine associated. Postmortem CT was carried out as a part of the routine forensic investigation, known as “Virtual Autopsy”. This included a noninvasive autopsy performed on a human body (developed in Switzerland). We enrolled 192 cases, 18–89 years of age, in whom a CT scan of the abdomen had been performed for forensic appraisal (2009–2010) [
RESULTS
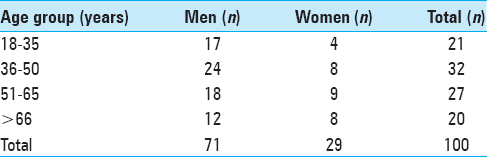
One hundred cases were included in this analysis; 71 were males and 29 were females. Details of age and sex distribution are outlined in
The narrowest point of the lateral diameter was found cranial of the hiatus sacralis in 46 cases and measured more than 13 mm in 95% of the cases. The surface areas of the sacral canal ranged 32–147 mm² and 26–159 mm² for females and males, respectively.
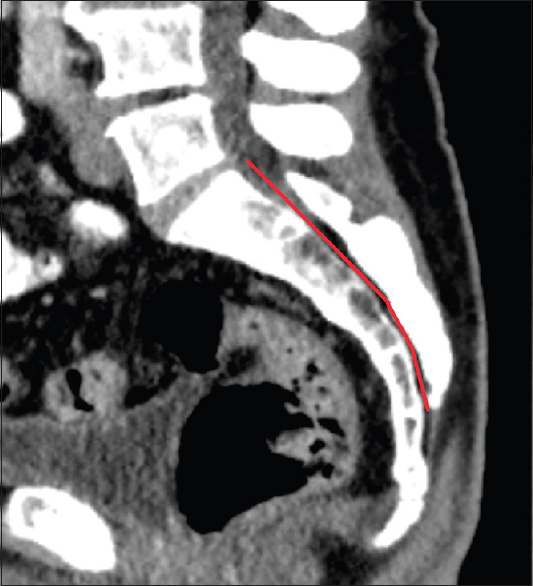
Regarding the angles of sacral kyphosis and lordosis see
DISCUSSION
Measurement of the anteroposterior diameter
In an autopsy specimen study by Witte et al.,[
Measurement of the lateral diameter
The lateral diameter in the Witte et al. study measured 14 mm ± 5 mm at the upper rim of S1 and 8 mm ± 5 mm at S4. The distance from the lumbosacral transition to the hiatus sacralis ranged from 85 to 155 mm. They also recorded the degrees of bending of the sacral canal up to 60°. It is yet unclear whether these findings in the geriatric population are applicable to the general population, as degenerative changes might have influenced the dimensions of the sacral canal. Moreover, materials based on autopsy specimens might contain artifacts that jeopardize the conclusions due to manipulation and dissection of the bony structures. To work more precisely and to get closer to the in-vivo status, our measurements were based on CT scans instead of measuring by millimeter grids.
However, our measurements were, as mentioned above, made in the supine position. As anatomy most often is position-dependent, measures of the lumbosacral angle might differ in prone position perioperatively.
Suggested use of flexible endoscope
Based on their findings, Witte et al. suggested the use of a flexible circular-shaped endoscope not exceeding a diameter of 4 mm. Due to their measurement and a trial of an arthroscope in five cadavers, they found that an endoscope of 4 mm would require surgical enlargement of the sacral canal to obtain access to the spinal canal in at least 20% of the patients.
Due to our findings of anatomical conditions and to accommodate 95% of the patients we suggest a flat-shaped endoscope having a vertical height of less than 2.5 mm, a width of less than 13 mm, and a minimal length of 290 mm.
Navigation issues with endoscopy
Several studies mentioned technical issues regarding navigation of the flexible but instable endoscope (2.5–3 mm diameter) through the spinal canal.[
Ruetten et al. also reported hemorrhages during mechanical processing, mostly due to surgical detachment of adhesions found in the dorsal epidural space of patients that had been previously operated upon.[
With respect to the anatomic conditions of the sacral canal and with consideration of the issues mentioned above, we believe the optimal endoscope should have a flat shape with similarly flattened instruments. Such geometry will provide better access to the spinal canal and will improve stability of the endoscope. A flexibility of 50° in the dorsal direction and 30° in the ventral direction would lead to better guidance. If in rare cases penetration into the spinal canal is interrupted due to a narrow hiatus sacralis, a simple enlargement of the hiatus by 1–2 cm in a cauda-cranial direction could be done to overcome the resistance. The dorsomedian connective tissue band fixing the dura mater to the flaval ligaments and narrowing the epidural space in the midline[
CONCLUSIONS
In conclusion, the hiatus sacralis presents a natural orifice for sacroscopy. It also offers an additional site for MIS. Our study delineates the requirement of a future sacral endoscope, as mentioned above. Further technical studies are warranted for the construction of an adequate endoscope.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Blomberg R. The dorsomedian connective tissue band in the lumbar epidural space of humans: An anatomical study using epiduroscopy in autopsy cases. Anesth Analg. 1986. 65: 747-52
2. Blomberg RG, Olsson SS. The lumbar epidural space in patients examined with epiduroscopy. Anesth Analg. 1989. 68: 157-60
3. Burman MS. Myeloscopy or the direct visualization of the spinal cord and its contents. J Bone Joint Surg. 1931. 13: 695-6
4. Fritsch EW, Heisel J, Rupp S. The failed back surgery syndrome: Reasons, intraoperative findings, and long-term results: A report of 182 operative treatments. Spine. 1996. 21: 626-33
5. Kotilainen E, Valtonen S. Clinical instability of the lumbar spine after microdiscectomy. Acta Neurochir (Wien). 1993. 125: 120-6
6. Ruetten S, Komp M, Merk H, Godolias G. Surgical treatment for lumbar lateral recess stenosis with the full-endoscopic interlaminar approach versus conventional microsurgical technique: A prospective, randomized, controlled study. J Neurosurg Spine. 2009. 10: 476-85
7. Ruetten S, Meyer O, Godolias G. [Epiduroscopic diagnosis and treatment of epidural adhesions in chronic back pain syndrome of patients with previous surgical treatment: First results of 31 interventions]. Z Für Orthop Ihre Grenzgeb. 2002. 140: 171-5
8. Ruetten S, Meyer O, Godolias G. Endoscopic surgery of the lumbar epidural space (epiduroscopy): Results of therapeutic intervention in 93 patients. Minim Invasive Neurosurg. 2003. 46: 1-4
9. Shimoji K, Fujioka H, Onodera M, Hokari T, Fukuda S, Fujiwara N. Observation of spinal canal and cisternae with the newly developed small-diameter, flexible fiberscopes. Anesthesiology. 1991. 75: 341-4
10. Sihvonen T, Herno A, Paljärvi L, Airaksinen O, Partanen J, Tapaninaho A. Local denervation atrophy of paraspinal muscles in postoperative failed back syndrome. Spine. 1993. 18: 575-81
11. Witte H, Hellweg S, Witte B, Grifka J. [Epiduroscopy with access via the sacral canal. Some constructional equipment requirements from the anatomic and biomechanical viewpoint]. Biomed Tech (Berl). 1997. 42: 24-9
12. Yadav YR, Parihar V, Namdev H, Agarwal M, Bhatele PR. Endoscopic interlaminar management of lumbar disc disease. J Neurol Surg Part Cent Eur Neurosurg. 2013. 74: 77-81
13. Yamada H, Yoshida M, Hashizume H, Minamide A, Nakagawa Y, Kawai M. Efficacy of novel minimally invasive surgery using spinal microendoscope for treating extraforaminal stenosis at the lumbosacral junction. J Spinal Disord Tech. 2012. 25: 268-76