- Department of Emergency and Organ Transplantation, University of Bari “Aldo Moro”, Bari, Italy
- Department of Veterinary Medicine, University of Perugia, Perugia, Italy
- Animal Pathology Section, School of Biosciences and Veterinary Medicine, University of Camerino, Matelica, Italy
- Department of Neurology, Public Health and Disability, Fondazione IRCCS Istituto Neurologico Carlo Besta, Milan, Italy
- Department of Neurosurgery, San Salvatore City Hospital, L'Aquila, Italy
- Department of Neurosurgery, Fondazione IRCCS Policlinico S. Matteo, Pavia, Italy
Correspondence Address:
Luzzi Sabino
Department of Emergency and Organ Transplantation, University of Bari “Aldo Moro”, Bari, Italy
DOI:10.4103/sni.sni_369_17
Copyright: © 2018 Surgical Neurology International This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Luzzi Sabino, Crovace Alberto Maria, Lacitignola Luca, Valentini Valerio, Francioso Edda, Rossi Giacomo, Invernici Gloria, Galzio Renato Juan, Crovace Antonio. Engraftment, neuroglial transdifferentiation and behavioral recovery after complete spinal cord transection in rats. 25-Jan-2018;9:19
How to cite this URL: Luzzi Sabino, Crovace Alberto Maria, Lacitignola Luca, Valentini Valerio, Francioso Edda, Rossi Giacomo, Invernici Gloria, Galzio Renato Juan, Crovace Antonio. Engraftment, neuroglial transdifferentiation and behavioral recovery after complete spinal cord transection in rats. 25-Jan-2018;9:19. Available from: http://surgicalneurologyint.com/?post_type=surgicalint_articles&p=8753
Abstract
Background:Proof of the efficacy and safety of a xenogeneic mesenchymal stem cell (MSCs) transplant for spinal cord injury (SCI) may theoretically widen the spectrum of possible grafts for neuroregeneration.
Methods:Twenty rats were submitted to complete spinal cord transection. Ovine bone marrow MSCs, retrovirally transfected with red fluorescent protein and not previously induced for neuroglial differentiation, were applied in 10 study rats (MSCG). Fibrin glue was injected in 10 control rats (FGG). All rats were evaluated on a weekly basis and scored using the Basso–Beattie–Bresnahan (BBB) locomotor scale for 10 weeks, when the collected data were statistically analyzed. The spinal cords were then harvested and analyzed with light microscopy, immunohistochemistry, and immunofluorescence.
Results:Ovine MSCs culture showed positivity for Nestin. MSCG had a significant and durable recovery of motor functions (P <.001 red fluorescence was found at the injury sites in mscg. positivity for nestin tubulin ng2 glia neuron-specific enolase vimentin and kd neurofilament were also same sites.>
Conclusions:Xenogeneic ovine bone marrow MSCs proved capable of engrafting into the injured rat spinal cord. Transdifferentiation into a neuroglial phenotype was able to support partial functional recovery.
Keywords: Mesenchymal stem cells, neuroglial differentiation, neurological recovery, red fluorescent protein, spinal cord injury
INTRODUCTION
Spinal cord injury (SCI) is a devastating condition affecting millions of people every year worldwide, especially young males. The overall number of paraplegic and quadriplegic people living in the U.S. is about 1.5 million, with an annual global cost for the healthcare system exceeding $10 billion.[
Mesenchymal stem cells (MSC) are potential candidates for a possible cell-based cure of SCI[
The present study aims at assessing the potential for engraftment, neurodifferentiation, and functional neuroglial regeneration following xenogeneic ovine bone marrow-derived MSCs in a rat model of complete spinal cord transection.
MATERIALS AND METHODS
Study design
All experiments were approved by the ethical committee of the University of Bari (Aldo Moro), Italy, in accordance with the national animal welfare legislation and in compliance with the guidelines outlined in the NRC Guide for the Care and Use of Laboratory Animals.
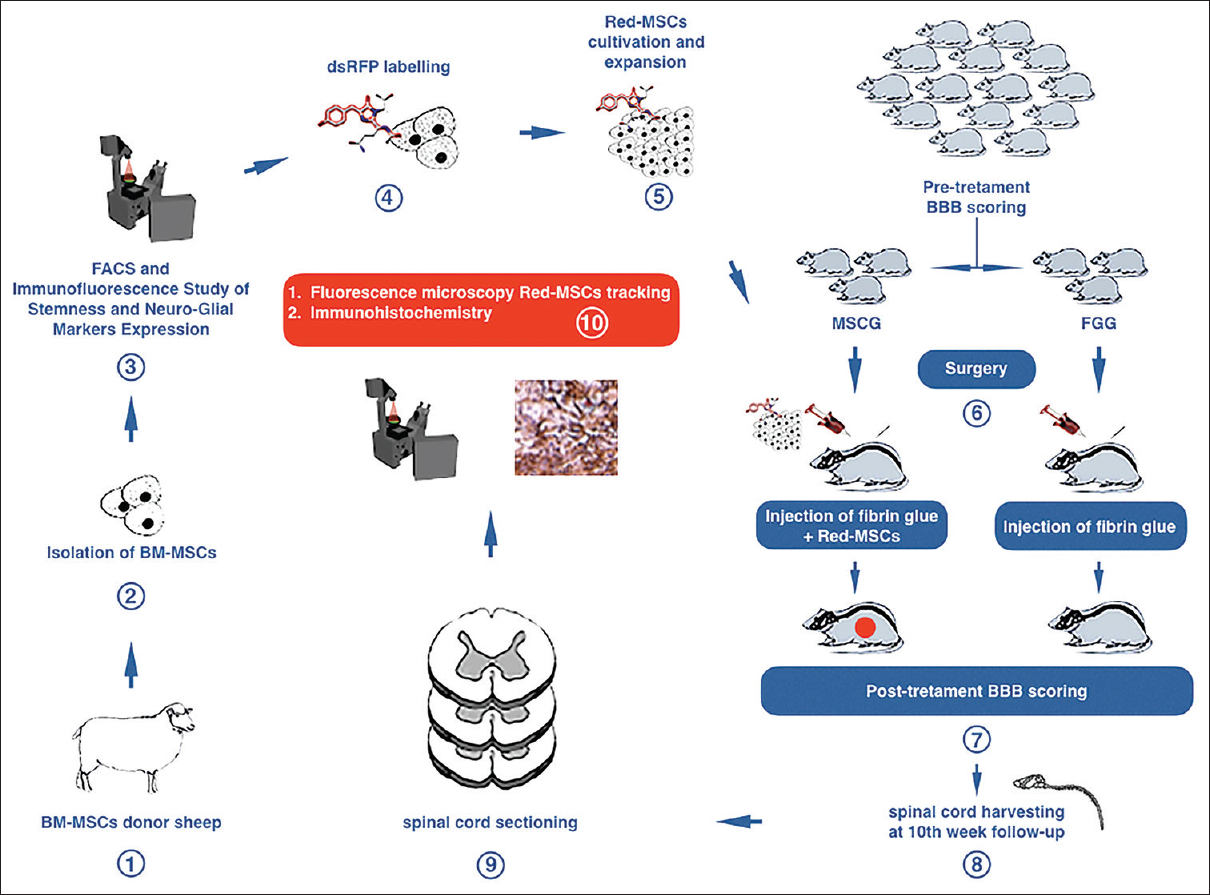
The first step of the study protocol involved the collection, isolation, and culture of bone marrow MSCs from healthy donor sheep and immunofluorescence analysis of immature neuroglial markers expression. MSCs were then retrovirally transfected with Red Fluorescent Protein (dsRFP) gene. Transfection with dsRFP would enable the cell to synthesize the marker as evidence of engraftment into the injured rat spinal cord. Subsequently, 20 Wistar rats underwent a pretreatment behavioral assessment by means of the Basso–Beattie–Bresnahan scoring system (BBB score)[
Figure 1
Diagram showing the design and experiments of the study. BM-MSCs: bone marrow mesenchymal stem cells; FACS: Fluorescence Activated Cell Sorting; Red-MSCs: mesenchymal stem cells retrovirally transfected with dsRFP gene; BBB: Basso-Beattie-Bresnahan score; MSCG: Mesenchymal Stem Cells Group; FGG: Fibrin Glue Group
Xenogeneic ovine bone marrow collection and isolation and culture of MSCs
A healthy donor sheep (Bergamasca breed, 2 year-old females, 45 kg in weight, not inbred) was selected as the source of MSCs. The donor sheep was sedated with diazepam (0.25 mg/kg) and the area of the tuber coxae was surgically prepared. Lidocaine (2%, 20 mL) was injected subcutaneously. A 14-gauge Jamshidi needle was inserted into the tuber coxae, and a 50 ml heparinized syringe was used to collect 30 ml of bone marrow. Bone marrow mononuclear cells were isolated on a Biocoll separating solution by centrifugation at 425 g for 30 minutes. The separated cells were counted with a nuclear stain (0.1% methyl violet in 0.1 M citric acid), rinsed twice with phosphate buffered saline (PBS), and suspended in an adequate amount of fibrin glue (Tisseel Baxter BioScience™, Munich, Germany). Cells were incubated at 37°C in a humidified 5% CO2 atmosphere for 2 weeks, replacing the medium twice a week. The cells were then washed with PBS, pH 7.2, fixed with buffered 4% formalin, and stained with 1% methylene blue in borate buffer (10 mM, pH 8.8). The mononuclear bone marrow cells were isolated as described above and seeded in flasks at a concentration of 4–5 × 106 cells/cm2 in complete Coon's medium at 37°C in a humidified 5% CO2 atmosphere. The medium was replaced twice a week until the cells reached 30% confluence.
Fluorescence activated cell sorting
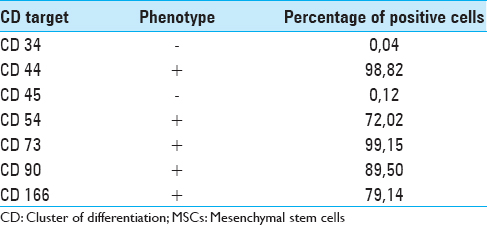
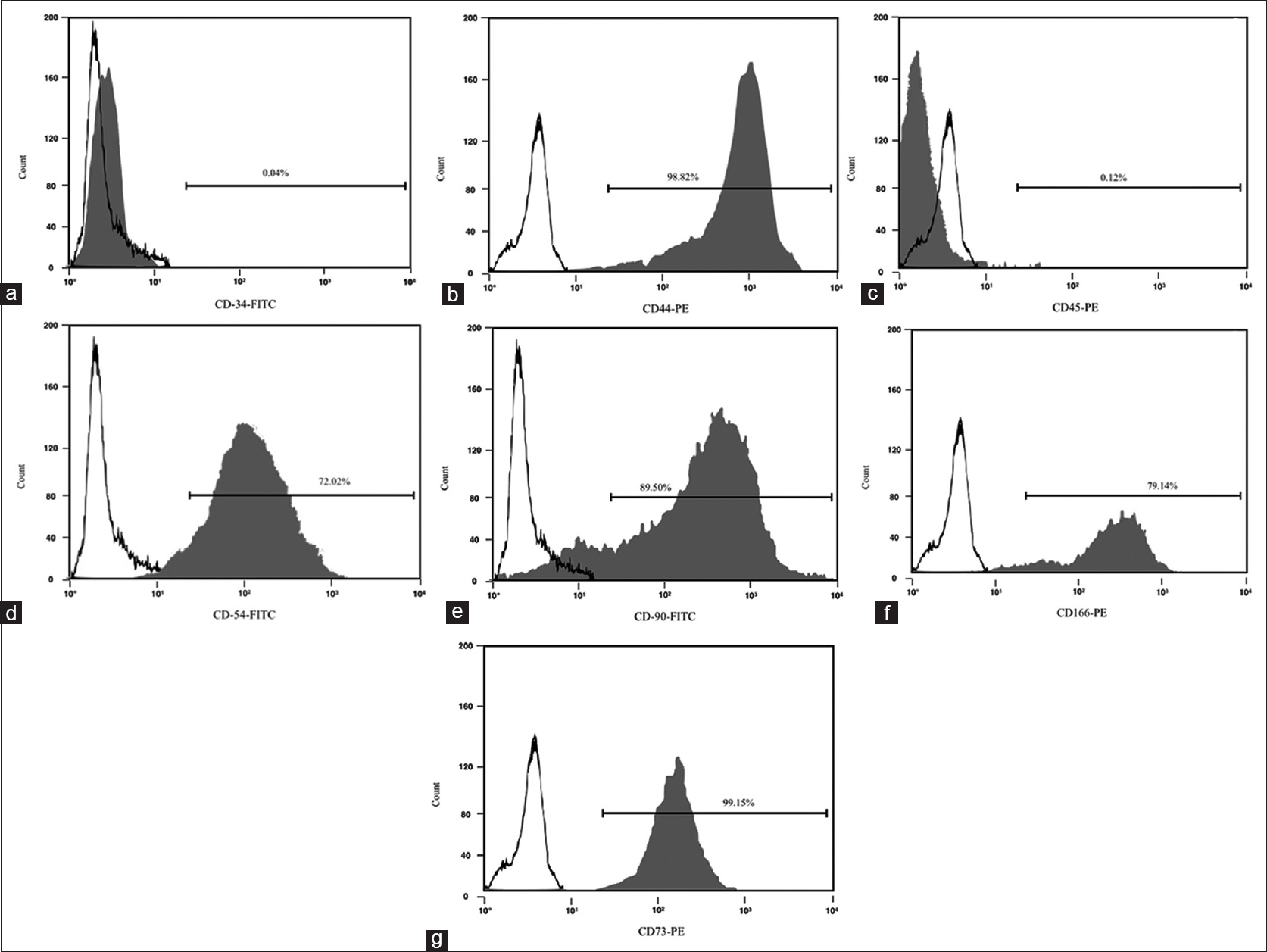
Fluorescence activated cell sorting (FACS) was employed to assess the immunophenotype of MSCs in terms of cluster of differentiation (CD). The list of the CD targets selected to assess the native immunophenotype of the ovine bone marrow MSCs, with the relative percentage of positivity for each tested CD, is presented in
Immunofluorescence cytochemical study of native neuroglial markers expression in MSCs
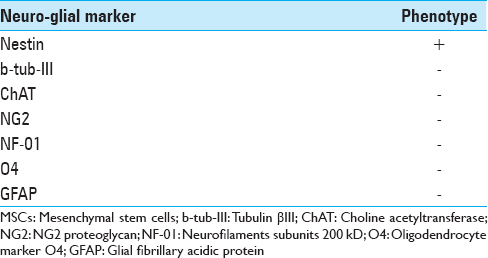
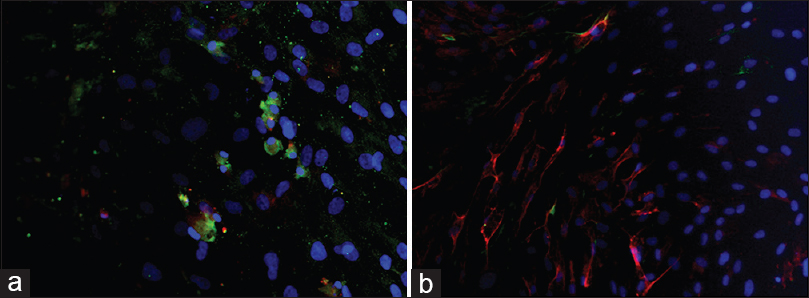
The cells were fixed for 20 min in 4% paraformaldehyde in phosphate-buffered saline (pH 7·4), and then washed and incubated for 90 min at 37°C with PBS plus 0.3% Triton containing 10% normal goat serum and the appropriate antibody mixture. The specific antibodies used to detect the antigens were anti-human glial fibrillary acidic protein (GFAP), NG2, mouse anti-human tubulin βIII (b-tub-III), Nestin (Chemicon, Temecula, CA, USA), rabbit anti-Neurofilaments subunits 200 kD (NF-01), rabbit anti-choline acetyltransferase (ChAT) (Sigma-Aldrich, St. Louis, MO, USA), mouse anti-oligodendrocyte marker O4 (O4) (R&D Systems, MN, USA). The cells’ culture was then washed twice with PBS and incubated with an appropriate secondary antibody: anti-rabbit IgG (1:1000), anti-mouse IgG (1:500) (Cy2 and Cy3; Jackson Immunoresearch) for 45 min at room temperature. The cells were incubated with 4,6- diamidino-2-phenylinole dihydrochloride (DAPI) (1 g/L in methanol, 15 min at 37°C) (Sigma-Aldrich, St. Louis, MO, USA) and Fluorsave™ (Calbiochem, La Jolla, USA) and viewed under a Zeiss Axiophot-2 microscope.
The list of early neuronal and glial targets tested on MSCs before the transplant is shown in
dsRFP cells labeling
dsRFP gene was retrovirally transfected into MSCs cultures by Dr. Andrea Levi at the Neurobiology and Molecular Medicine Branch of C.N.R. (Centro Nazionale delle Ricerche), Rome, Italy. The retrovirus coding for dsRFP (Retro V-RFP) was added along with 200 μL of serum-free medium, and the flask was shaken gently every 15 minutes for 2 hours. The passage 5 MSCs were transfected with the recombinant replication-defective retrovirus at different multiplicities of infection in the range of 0–200 units. After incubation with Retro V-RFP for 2 hours, culture medium containing 10% fetal bovine serum was added into the flask. The transfected MSCs were cultured for 72 hours and then microscopically observed. Three high-power fields of view were selected randomly to count the cells positive for retroviral transfection. The estimated transfection rate was 88.3%. The cells obtained by retroviral transfection of dsRFP gene were referred as Red-MSCs.
Pretreatment behavioral appraisal
Twenty rats (Wistar, 200–250 g) of either sex, sex not affecting the potential for neurologic recovery in SCI, were evaluated for behavioral analysis by the BBB open field locomotor scale.[
Xenogeneic MSCs transplantation
The rats were anesthetized according to a standard protocol involving the intraperitoneal administration of Fentanyl/Fluanisone (0.3 mg/kg i.p., Hypnorm, Janssen, Belgium) and Midazolam (0.6 mg/kg i.p., Dormicum, Roche, Switzerland), then shaved, placed in a heating pad, achieving a body temperature at 37°C during the entire length of surgery, and underwent a standard mid-thoracic two-level laminectomy (T8-T9). The dura was carefully opened and the spinal cord was exposed. Under microscopic view (4×), a complete transection of the cord was performed making a single knife cut with a standard surgical scalpel blade no. 11 mounted on a Bard Parker surgical blade handle #3. All the cord transections were performed in an axial plane and according to the same standard technique in both groups. Thereafter, a solution containing 6 × 106 cells/mL Red-MSCs and fibrin glue (Tisseel, Baxter BioScience™) was applied at the cord transection level using a microsyringe in the MSCG group. Conversely, only fibrin glue was applied in the FGG rats. The dura was then closed in a running watertight fashion to avoid early or late central nervous system infections; muscles and skin were re-approximated with interrupted sutures [
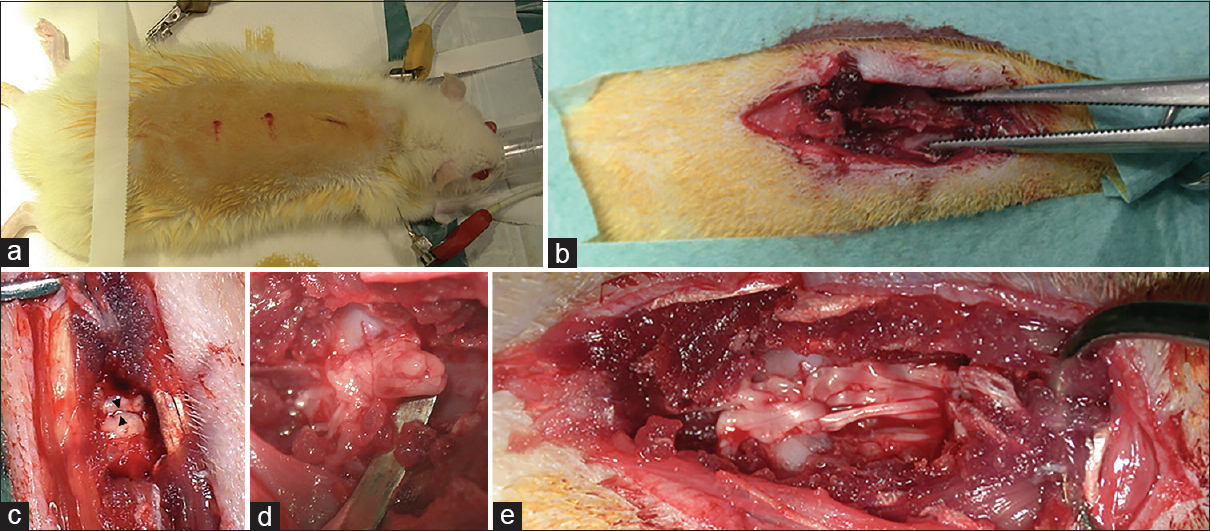
Figure 2
Rat spinal cord transection. (a) preparation and shaving of the rat; (b) skeletonization of the spine for mid-thoracic laminectomy; (c and d) spinal cord transection; (e) release of the solution containing Red-MSCs and fibrin glue at the injury site in MSCG. In some cases, the permeation of the fibrin glue caused a partial dissociation of the main bundles of the spinal cord
A standard postoperative analgesic protocol involving the administration of Buprenorphine (0.03 mg/kg, s.c., Temgesic, Reckitt & Colman, UK) was enacted during the early postoperative period. All the rats were evaluated for behavioral recovery within 24 hours of surgery (T-post), and housed under a 12:12-h light/dark cycle with water and food ad libitum. Careful monitoring of rats with daily weight recordings and bladder expression allowed for early detection of postoperative complications. Trimethoprim (0.83 ml/kg, i.p., Bactrim, Roche, Switzerland) was promptly started in case of infection.
Posttreatment behavioral follow-up
In addition to early postoperative evaluation (T-post), all the rats were evaluated weekly until the 70th posttreatment day. A short digital
Light microscopy
Within 1 week from the last clinical and behavioral evaluation, all the rats were sacrificed with a pentobarbital overdose (50 mg/100 g), and all the previously injured spinal cords were harvested. Each cord was divided into three equal blocks of 5 mm in length. The first block was harvested at the maximal injury zone, whereas the other two blocks were sampled just above and below the cord transection.
Each block was fixed in 10% neutral buffered formalin for 48 hours. The cords were then washed, dehydrated in a series of passages through ethanol solutions at different concentrations (40% to 100%) and embedded in paraffin wax. The specimens were frozen in isopentane at −40°C and then cut in sections of 3 m thickness. The sections were then stained with hematoxylin-eosin (HE) for pathological examination. A series of digital photographs was obtained for both groups.
dsRFP cells tracking
In the MSCG, Red-MSCs tracking was achieved by fluorescence microscopy (Olympus BX61) at a 20 × magnification (absorption/emission peak 458 nm and 583 nm, respectively). The total amount of fluorescence was measured for each field at the same magnification by commercially available software (Image J, Windows Excel Microsoft Corporation). For each field, the background fluorescence was subtracted to avoid any potentially biasing factor and to obtain the real total fluorescence of the specimen. Images were digitally recorded.
Immunohistochemistry
Immunohistochemical study of the MSCG spinal cord specimens was performed to confirm the presence – and thus putative engraftment – of the xenogeneic bone marrow MSCs at the injury site. On the basis of the already reported immunohistochemical phenotype of ovine bone marrow MSCs,[
RESULTS
Evidence of stemness and native immunophenotype of MSCs
Immunostaining positivity for CD 44, CD 54, CD 73, CD 90 and CD 166 was found in ovine MSCs culture. A lack of expression of CD 34 and CD 45 confirmed the stemness of these cells [
Surgery
All surgeries were performed successfully. Particularly, the use of a very sharp knife (blade no. 11), together with a microscopic detailed visualization of the surgical field, allowed micrometric axial cuts of the spinal cords: the gap between the proximal and distal stumps of the cord never exceed 4 mm in length. This aspect was considered paramount to minimize damage of the cord, both at the white matter and gray matter level, and, ultimately, to create the anatomical assumptions for the regrowth of neurites out of the gray matter core which is the vital component of the so-called cortico-trunco-reticulo-proprio-spinal pathway.[5–7] The assessment of the completeness of the spinal cord transection was achieved intraoperatively under microscopic vision by gentle elevation of the two stumps with a Penfield n. 2 periosteal elevator, as shown in
Posttreatment behavioral appraisal
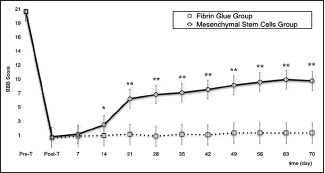
Preoperatively, all rats showed a normal behavioral score; immediately after surgery, the BBB score dramatically decreased to a near zero level in both groups as an expression of the completeness of the lesional surgery.
In the MSCG, in the third week, one rat was lost due to severe self-mutilations. At the ninth week, two rats reached a score of 14 consisting of plantar stepping with full weight support and complete forelimb–hindlimb coordination[
In the FGG, two rats were lost in the second week and a another in the fourth week, all because of self-mutilations. The remaining rats did not experience a significant functional recovery [
Statistical analysis
In all treated rats, a difference between T-pre and T-post BBB scores was found (P <.001). Comparison between MSCG and FGG evinced a difference in terms of functional recovery of the MSCG over the FGG starting from the 14th day of behavioral assessment (P <.05). This difference between groups was confirmed throughout the follow-up (P <.001) [
Light microscopy
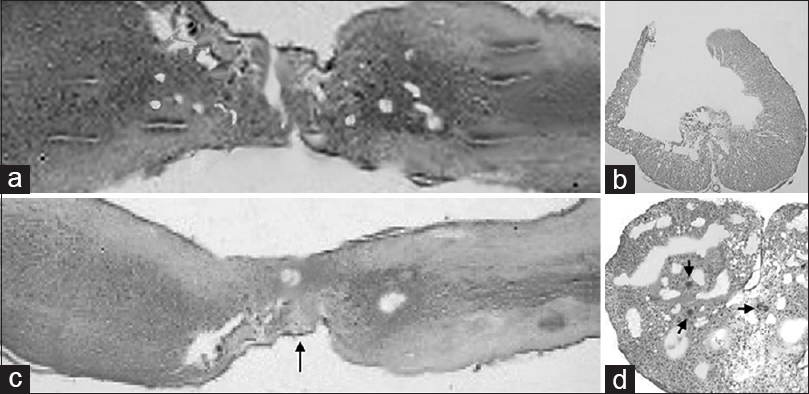
In the group of rats treated exclusively with fibrin glue injection, a general nonspecific pattern of severe glial scarring with scattered and necrotic large nucleated neurons was found. The scar tended to form bridges between the previously transected spinal cord stumps. It involved the entire axial plane of the cord up to 1 cm from the injury site [Figure
Figure 5
Light microscopy in FGG (a, b) vs. MSCG (c and d) at the injury site 10 weeks after injury. (a) FGG sagittal (HE scale bars = 400 μm); (b) FGG axial cut at the level of glial scar (HE scale bars = 200 μm); (c) MSCG sagittal (HE scale bars = 400 μm). Black arrow indicates the neuroglial bridges between the spinal cord stumps at the injury site. (d) MSCG axial (HE scale bars = 200 μm). Black arrows show a tight distribution of the neuroglial cells around the vessels
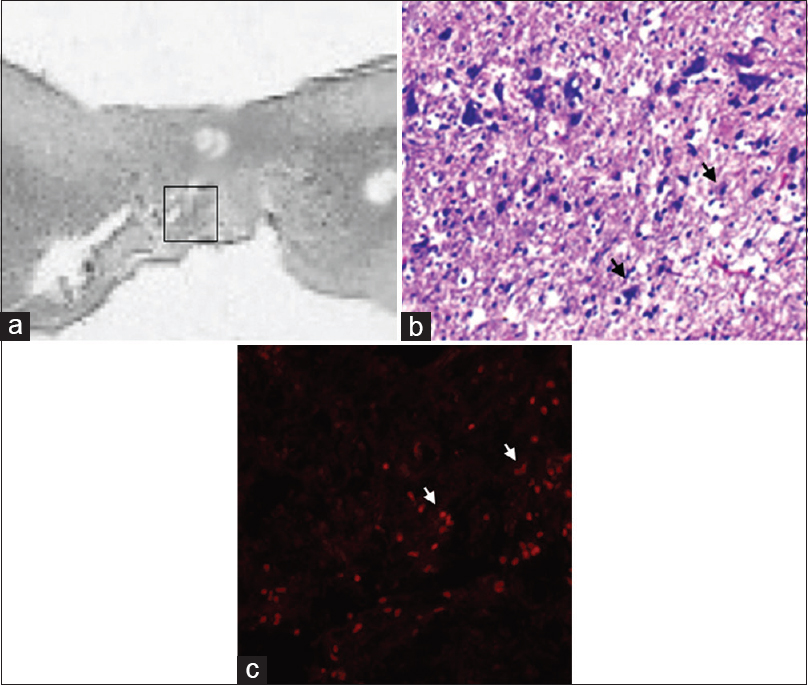
In the MSCG, HE staining revealed a tighter distribution of neuroglial cells, especially near the nerve stumps and around the vessels [Figure
Fluorescence microscopy
Fluorescent microscopy allowed clear tracking of the presence of red fluorescence emission, attributable to a full engraftment of the transplanted Red-MSCs, at the cord injury sites in the MSCG. Fluorescence emission was generally arranged in the form of clusters of multiple red spots, which perfectly matched with the distribution of the MSCs at the injury site [
Figure 6
(a) Sagittal section (HE scale bars = 400 μm) of the harvested rat spinal cord in MSCG at 10th week after injury. Squared area indicates the site of sampling. (b) distribution of MSCs (black arrows) at the injury site in the MSCG (HE × 40); (c) co-localizzation of dsRFP MSCs (white arrows) within the injury site (fluorescence microscopy, excitation light 568 nm)
Immunohistochemistry
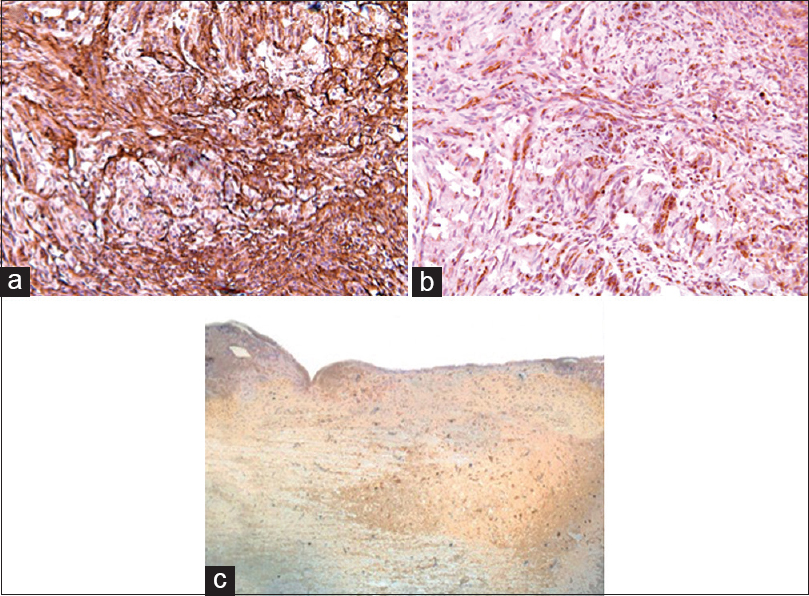
In the MSCG, positivity for Nestin, NG2, β-III tubulin, Vimentin, NF-01, and NSE immunostaining was found in all but two rats at the injury sites [
In the FGG, only very weak positivity for Vimentin was found probably associated with the fibroblastic component of the scar. No markers of neuroglial differentiation were detected in this group.
DISCUSSION
SCI involves both an acute and a chronic phase. The acute phase is characterized by blood vessels disruption, extensive posttraumatic cell death, recruitment of macrophages, neutrophils, and leukocytes tasked with cell debris phagocytosis and containment of further tissue damage.[
In 1980, Aguayo's group published a seminal work where they demonstrated, for the first time, the intrinsic ability the central nervous system (CNS) axons to regenerate and grow into the peripheral nerve system.[
A cell-based regenerative approach involves the transplantation of specific stem cell lines into the injured CNS tissue, followed by integration and possible differentiation into actual neurons or glia: this process would promote tissue restoration directly and indirectly by stemming inhibitory influences. Bone marrow-derived MSCs are ideal candidates in this regard.[
In the present study, we found that even the ovine xenogeneic bone marrow harvested MSCs proved to be extremely effective in promoting significant behavioral recovery. The red fluorescence emission found at the injury sites confirms the capacity of these cells to engraft within the damaged host neural tissue. Of relevance, we also found at the injury site immunopositivity for neural progenitors as Nestin, NG2 β-III tubulin, NSE, vimentin, and NF-01, undoubtedly an expression of an early “neurolike” and “glia-like” differentiation pattern.
Three important mechanisms seem to be responsible for the low immunogenicity of MSCs – lack of MHC-II and co-stimulatory molecule expression, modulation of dendritic cells, as well direct antagonization of NK, CD8+, and CD4+ T cell function, and induction of a local immunosuppressive microenvironment through the production of prostaglandins and interleukin-10.[
Our findings suggest that MSCs, including xenogeneic ovine MSCs, favorably alter post-SCI neural tissue disruption. Ovine xenogeneic MSCs are known to migrate across the blood–brain barrier, reconstitute the neuroglial pool, and differentiate into neurons-like and microglia-like cells.[
In our study, we have selected a complete transection model rather than a contusion (incomplete) model of SCI. This choice is justified by the willingness to better assess the actual neuroregenerative potential of xenogeneic ovine bone marrow-derived MSCs, together with their capacity to survive, engraft, and form “de novo” neuron-like or glial-like cells, thereby leading to neural tissue reconstitution.
Our approach is best conceptualized within the boundaries of the GEMINI spinal cord fusion protocol.[
MSC were applied immediately upon transection. As such they would perfectly fit inside the transection-reapposition treatment of chronic SCI (see 6) and could be applied in this context.
In conclusion, xenogeneic ovine bone marrow MSCs proved intrinsic, capable to survive, and engraft into the injured rat spinal cord; moreover, they showed signs of transdifferentiation into a neuroglial phenotype, and most importantly to support functional recovery.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Video available on: www.surgicalneurologyint.com
References
1. Assunção-Silva RC, Gomes ED, Sousa N, Silva NA, Salgado AJ. Hydrogels and Cell Based Therapies in Spinal Cord Injury Regeneration. Stem Cells Int. 2015. 2015: 948040-
2. Baptiste DC, Fehlings MG. Update on the treatment of spinal cord injury. Prog Brain Res. 2007. 161: 217-33
3. Basso DM, Beattie MS, Bresnahan JC. A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma. 1995. 12: 1-21
4. Benson MD, Romero MI, Lush ME, Lu QR, Henkemeyer M, Parada LF. Ephrin-B3 is a myelin-based inhibitor of neurite outgrowth. Proc Natl Acad Sci U S A. 2005. 102: 10694-9
5. Canavero S, Ren X-P. The Spark of Life: Engaging the Cortico-Truncoreticulo-Propriospinal Pathway by Electrical Stimulation. CNS Neurosci Ther. 2016. 22: 260-1
6. Canavero S, Ren X. Houston, GEMINI has landed: Spinal cord fusion achieved. Surg Neurol Int. 2016. 7: S626-8
7. Canavero S, Ren X, Kim C-Y, Rosati E. Neurologic foundations of spinal cord fusion (GEMINI). Surgery. 2016. 160: 11-9
8. Cao F, Feng S. Human umbilical cord mesenchymal stem cells and the treatment of spinal cord injury. Chin Med J (Engl). 2009. 122: 225-31
9. Caplan AI. Adult mesenchymal stem cells for tissue engineering versus regenerative medicine. J Cell Physiol. 2007. 213: 341-7
10. Chen MS, Huber AB, van der Haar ME, Frank M, Schnell L, Spillmann AA. Nogo-A is a myelin-associated neurite outgrowth inhibitor and an antigen for monoclonal antibody IN-1. Nature. 2000. 403: 434-9
11. Coleman WP, Benzel D, Cahill DW, Ducker T, Geisler F, Green B. A critical appraisal of the reporting of the National Acute Spinal Cord Injury Studies (II and III) of methylprednisolone in acute spinal cord injury. J Spinal Disord. 2000. 13: 185-99
12. Czernik M, Fidanza A, Sardi M, Galli C, Brunetti D, Malatesta D. Differentiation potential and GFP labeling of sheep bone marrow-derived mesenchymal stem cells. J Cell Biochem. 2013. 114: 134-43
13. Das AK, Gopurappilly R, Parhar I. Current status and prospective application of stem cell-based therapies for spinal cord injury. Curr Stem Cell Res Ther. 2011. 6: 93-104
14. Desantis S, Accogli G, Zizza S, Mastrodonato M, Blasi A, Francioso E. Ultrastructural study of cultured ovine bone marrow-derived mesenchymal stromal cells. Ann Anat. 2015. 201: 43-9
15. Dezawa M, Kanno H, Hoshino M, Cho H, Matsumoto N, Itokazu Y. Specific induction of neuronal cells from bone marrow stromal cells and application for autologous transplantation. J Clin Invest. 2004. 113: 1701-10
16. Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006. 8: 315-7
17. Eftekharpour E, Karimi-Abdolrezaee S, Fehlings MG. Current status of experimental cell replacement approaches to spinal cord injury. Neurosurg Focus. 2008. 24: E19-
18. Faden AI, Jacobs TP, Holaday JW. Comparison of early and late naloxone treatment in experimental spinal injury. Neurology. 1982. 32: 677-81
19. Friedenstein AJ, Chailakhjan RK, Lalykina KS. The development of fibroblast colonies in monolayer cultures of guinea-pig bone marrow and spleen cells. Cell Tissue Kinet. 1970. 3: 393-403
20. Goldschlager T, Oehme D, Ghosh P, Zannettino A, Rosenfeld JV, Jenkin G. Current and future applications for stem cell therapies in spine surgery. Curr Stem Cell Res Ther. 2013. 8: 381-93
21. Hall ED, Yonkers PA, Taylor BM, Sun FF. Lack of effect of postinjury treatment with methylprednisolone or tirilazad mesylate on the increase in eicosanoid levels in the acutely injured cat spinal cord. J Neurotrauma. 1995. 12: 245-56
22. Hall ED, Springer JE. Neuroprotection and acute spinal cord injury: A reappraisal. NeuroRx. 2004. 1: 80-100
23. Harrop JS, Hashimoto R, Norvell D, Raich A, Aarabi B, Grossman RG. Evaluation of clinical experience using cell-based therapies in patients with spinal cord injury: A systematic review. J Neurosurg Spine. 2012. 17: 230-46
24. Hata K, Fujitani M, Yasuda Y, Doya H, Saito T, Yamagishi S. RGMa inhibition promotes axonal growth and recovery after spinal cord injury. J Cell Biol. 2006. 173: 47-58
25. Holden C, Vogel G. Stem cells? Plasticity: Time for a reappraisal. Science. 2002. 296: 2126-9
26. Hurlbert RJ. Methylprednisolone for acute spinal cord injury: An inappropriate standard of care. J Neurosurg. 2000. 93: 1-7
27. Ito Y, Sugimoto Y, Tomioka M, Kai N, Tanaka M. Does high dose methylprednisolone sodium succinate really improve neurological status in patient with acute cervical cord injury.: A prospective study about neurological recovery and early complications?. Spine (Phila Pa 1976). 2009. 34: 2121-4
28. Jiang Y, Jahagirdar BN, Reinhardt RL, Schwartz RE, Keene CD, Ortiz-Gonzalez XR. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature. 2002. 418: 41-9
29. Joyce N, Annett G, Wirthlin L, Olson S, Bauer G, Nolta JA. Mesenchymal stem cells for the treatment of neurodegenerative disease. Regen Med. 2010. 5: 933-46
30. Karimi-Abdolrezaee S, Eftekharpour E. Stem cells and spinal cord injury repair. Adv Exp Med Biol. 2012. 760: 53-73
31. Koda M, Okada S, Nakayama T, Koshizuka S, Kamada T, Nishio Y. Hematopoietic stem cell and marrow stromal cell for spinal cord injury in mice. Neuroreport. 2005. 16: 1763-7
32. Koshizuka S, Okada S, Okawa A, Koda M, Murasawa M, Hashimoto M. Transplanted hematopoietic stem cells from bone marrow differentiate into neural lineage cells and promote functional recovery after spinal cord injury in mice. J Neuropathol Exp Neurol. 2004. 63: 64-72
33. Louro J, Pearse DD. Stem and progenitor cell therapies: Recent progress for spinal cord injury repair. Neurol Res. 2008. 30: 5-16
34. Löw K, Culbertson M, Bradke F, Tessier-Lavigne M, Tuszynski MH. Netrin-1 is a novel myelin-associated inhibitor to axon growth. J Neurosci. 2008. 28: 1099-108
35. Lyahyai J, Mediano DR, Ranera B, Sanz A, Remacha AR, Bolea R. Isolation and characterization of ovine mesenchymal stem cells derived from peripheral blood. BMC Vet Res. 2012. 8: 169-
36. Makino S, Fukuda K, Miyoshi S, Konishi F, Kodama H, Pan J. Cardiomyocytes can be generated from marrow stromal cells in vitro . J Clin Invest. 1999. 103: 697-705
37. Matsumoto T, Tamaki T, Kawakami M, Yoshida M, Ando M, Yamada H. Early complications of high-dose methylprednisolone sodium succinate treatment in the follow-up of acute cervical spinal cord injury. Spine (Phila Pa 1976). 2001. 26: 426-30
38. McCreedy DA, Sakiyama-Elbert SE. Combination therapies in the CNS: Engineering the environment. Neurosci Lett. 2012. 519: 115-21
39. McKerracher L, David S, Jackson DL, Kottis V, Dunn RJ, Braun PE. Identification of myelin-associated glycoprotein as a major myelin-derived inhibitor of neurite growth. Neuron. 1994. 13: 805-11
40. Meirelles L da S, Nardi NB. Methodology, biology and clinical applications of mesenchymal stem cells. Front Biosci. 2009. 14: 4281-98
41. Miller RH, Bai L. Translating stem cell therapies to the clinic. Neurosci Lett. 2012. 519: 87-92
42. Moreau-Fauvarque C, Kumanogoh A, Camand E, Jaillard C, Barbin G, Boquet I. The transmembrane semaphorin Sema4D/CD100, an inhibitor of axonal growth, is expressed on oligodendrocytes and upregulated after CNS lesion. J Neurosci. 2003. 23: 9229-39
43. Mukhopadhyay G, Doherty P, Walsh FS, Crocker PR, Filbin MT. A novel role for myelin-associated glycoprotein as an inhibitor of axonal regeneration. Neuron. 1994. 13: 757-67
44. Nandoe Tewarie RS, Hurtado A, Bartels RH, Grotenhuis A, Oudega M. Stem cell-based therapies for spinal cord injury. J Spinal Cord Med. 2009. 32: 105-14
45. Noble M, Mayer-Pröschel M, Davies JE, Davies SJA, Pröschel C. Cell therapies for the central nervous system: How do we identify the best candidates?. Curr Opin Neurol. 2011. 24: 570-6
46. Obermair F-J, Schröter A, Thallmair M. Endogenous neural progenitor cells as therapeutic target after spinal cord injury. Physiology (Bethesda). 2008. 23: 296-304
47. Phinney DG, Prockop DJ. Concise review: Mesenchymal stem/multipotent stromal cells: The state of transdifferentiation and modes of tissue repair--current views. Stem Cells. 2007. 25: 2896-902
48. Prockop DJ. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science. 1997. 276: 71-4
49. Reynolds BA, Weiss S. Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science. 1992. 255: 1707-10
50. Richardson PM, McGuinness UM, Aguayo AJ. Axons from CNS neurons regenerate into PNS grafts. Nature. 1980. 284: 264-5
51. Rowland JW, Hawryluk GWJ, Kwon B, Fehlings MG. Current status of acute spinal cord injury pathophysiology and emerging therapies: Promise on the horizon. Neurosurg Focus. 2008. 25: E2-
52. Ruff CA, Wilcox JT, Fehlings MG. Cell-based transplantation strategies to promote plasticity following spinal cord injury. Exp Neurol. 2012. 235: 78-90
53. Ryan JM, Barry F, Murphy JM, Mahon BP. Interferon-gamma does not break, but promotes the immunosuppressive capacity of adult human mesenchymal stem cells. Clin Exp Immunol. 2007. 149: 353-63
54. Sakaguchi Y, Sekiya I, Yagishita K, Muneta T. Comparison of human stem cells derived from various mesenchymal tissues: Superiority of synovium as a cell source. Arthritis Rheum. 2005. 52: 2521-9
55. Sakiyama-Elbert SE, Panitch A, Hubbell JA. Development of growth factor fusion proteins for cell-triggered drug delivery. FASEB J. 2001. 15: 1300-2
56. Sanchez-Ramos J, Song S, Cardozo-Pelaez F, Hazzi C, Stedeford T, Willing A. Adult bone marrow stromal cells differentiate into neural cells in vitro . Exp Neurol. 2000. 164: 247-56
57. Sekhon LH, Fehlings MG. Epidemiology, demographics, and pathophysiology of acute spinal cord injury. Spine (Phila 1976). 2001. 26: S2-12
58. Short DJ, El Masry WS, Jones PW. High dose methylprednisolone in the management of acute spinal cord injury-a systematic review from a clinical perspective. Spinal Cord. 2000. 38: 273-86
59. Silva NA, Sousa N, Reis RL, Salgado AJ. From basics to clinical: A comprehensive review on spinal cord injury. Prog Neurobiol. 2014. 114: 25-57
60. Silver J, Miller JH. Regeneration beyond the glial scar. Nat Rev Neurosci. 2004. 5: 146-56
61. Simard AR, Rivest S. Bone marrow stem cells have the ability to populate the entire central nervous system into fully differentiated parenchymal microglia. FASEB J. 2004. 18: 998-1000
62. Stenderup K, Justesen J, Clausen C, Kassem M. Aging is associated with decreased maximal life span and accelerated senescence of bone marrow stromal cells. Bone. 2003. 33: 919-26
63. Stocum DL. Regenerative biology and medicine. J Musculoskelet Neuronal Interact. 2002. 2: 270-3
64. Takayama T, Kondo T, Kobayashi M, Ohta K, Ishibashi Y, Kanemaru T. Characteristic morphology and distribution of bone marrow derived cells in the cornea. Anat Rec (Hoboken). 2009. 292: 756-63
65. Vawda R, Fehlings MG. Mesenchymal cells in the treatment of spinal cord injury: Current and future perspectives. Curr Stem Cell Res Ther. 2013. 8: 25-38
66. Wang KC, Koprivica V, Kim JA, Sivasankaran R, Guo Y, Neve RL. Oligodendrocyte-myelin glycoprotein is a Nogo receptor ligand that inhibits neurite outgrowth. Nature. 2002. 417: 941-4
67. Weiss S, Dunne C, Hewson J, Wohl C, Wheatley M, Peterson AC. Multipotent CNS stem cells are present in the adult mammalian spinal cord and ventricular neuroaxis. J Neurosci. 1996. 16: 7599-609
68. Wyatt LA, Keirstead HS. Stem cell-based treatments for spinal cord injury. Prog Brain Res. 2012. 201: 233-52
69. Zuk PA, Zhu M, Ashjian P, De Ugarte DA, Huang JI, Mizuno H. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002. 13: 4279-95