- Department of Neurosurgery, Maastricht University Medical Center, Maastricht, The Netherlands
- Department of Nose and Throat/Head and Neck Surgery, Maastricht University Medical Center, Maastricht, The Netherlands
- Donders Institute for Brain, Cognition and Behaviour, Radboud University Medical Center, Nijmegen, The Netherlands
Correspondence Address:
Yasin Temel
Department of Neurosurgery, Maastricht University Medical Center, Maastricht, The Netherlands
Donders Institute for Brain, Cognition and Behaviour, Radboud University Medical Center, Nijmegen, The Netherlands
DOI:10.4103/sni.sni_106_17
Copyright: © 2017 Surgical Neurology International This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Saeed Banaama, Robert Stokroos, Youssef Yakkioui, Jacobus van Overbeeke, Yasin Temel. A novel drainage approach in patients with cholesterol granuloma: From petrous apex to mastoid air cell. 22-Aug-2017;8:196
How to cite this URL: Saeed Banaama, Robert Stokroos, Youssef Yakkioui, Jacobus van Overbeeke, Yasin Temel. A novel drainage approach in patients with cholesterol granuloma: From petrous apex to mastoid air cell. 22-Aug-2017;8:196. Available from: http://surgicalneurologyint.com/surgicalint-articles/a-novel-drainage-approach-in-patients-with-cholesterol-granuloma-from-petrous-apex-to-mastoid-air-cell/
Abstract
Background:Cholesterol granulomas (CG) of the petrous apex (CGPA) are benign lesions that have high recurrence rates after surgical intervention. We describe the use of a robust silicon drain between the petrous apex and mastoid air cells to allow constant aeration of the lesion for preventing recurrence.
Case Description:A retrospective analysis was performed using the data of four patients treated at the Maastricht University Medical Centre between 2014 and 2016. Using the middle fossa approach, the petrous apex was reached, the cyst was opened, and the content aspirated. Subsequently, a robust silicon drain was placed between the cyst and mastoid air cell system. The outcome measures were clinical improvement of the symptoms and radiological parameters. The patients were female (n = 2) and male (n = 2) with an age range between 33 and 53 years at the time of the operation. Computed tomography and magnetic resonance imaging scans were used to confirm CG diagnosis. The most common presenting symptoms in our population were diplopia and headaches. The symptoms improved after surgery and there were no complications. Thus far, no recurrence has been observed and imaging shows aeration in the lesion area.
Conclusion:The use of a robust drain seems to be an effective, safe, and feasible option to prevent recurrences in patients with CG.
Keywords: Cholesterol granuloma, drainage, mastoid, petrous apex
INTRODUCTION
Cholesterol granulomas (CG) are rare and slowly growing cystic lesions surrounded by fibrous tissue. These lesions are formed via the reaction of foreign body giant cells against cholesterol crystals.[
Symptoms of CGs vary based on the location, size, and involvement of surrounding anatomical structures. Most lesions become symptomatic when they compress the adjoining structures, usually the cranial nerves V, VI, VII, or VIII. As a result, presenting symptoms are often related to a cranial nerve function deficit and include trigeminal neuralgia, diplopia, facial weakness, facial spasms, deafness, vertigo, tinnitus, headaches, and/or seizures.[
Computed tomography (CT) images show a well-defined expansive and erosive lesion with a density similar to that of the brain. On magnetic resonance imaging (MRI), the lesions appear with high intensity signal on both T1 and T2-weighted images due to presence of cholesterol. The rim of the lesion on T2-weighted imaging appears with a low intensity signal because of hemosiderin. No attenuation is seen on fluid attenuated inversion recovery (FLAIR) sequences. Apparent diffusion coefficient (ADC) sequences show no restriction of diffusion.[
When a lesion becomes symptomatic, surgical intervention is the preferred management strategy.[
METHODS AND RESULTS
Patients with the diagnosis of petrous apex CG who were referred to our Skull Base surgery team at the Maastricht University Medical center (MUMC+) in the Netherlands were included in this study after informed consent had been obtained. Due to the small sample size, formal statistical analyses were not performed. Detailed documentation on clinical and surgical information, complications, pre- and postoperative imaging findings, revision surgery, and audiometric data were analyzed.
Surgical procedure
The cyst was reached via a middle fossa extradural approach, unroofed by drilling and aspirated.[
CASES DESCRIPTIONS
The cases are briefly described below. No major comorbidities were present in the patients described here. The order of the cases is based on the duration of follow-ups.
Case 1
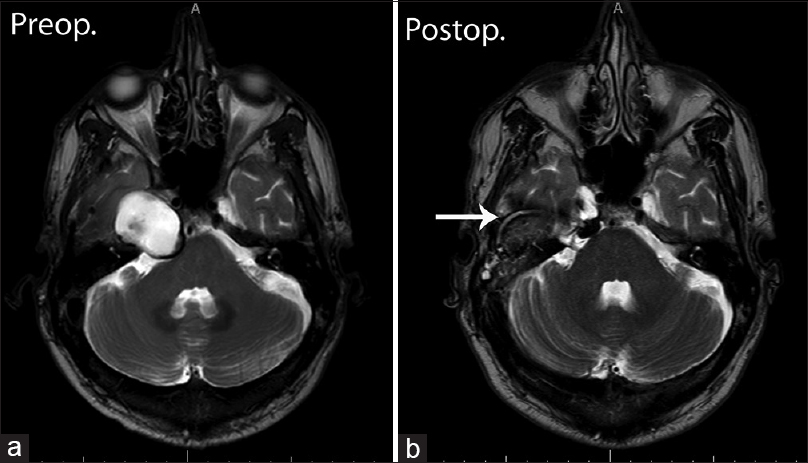
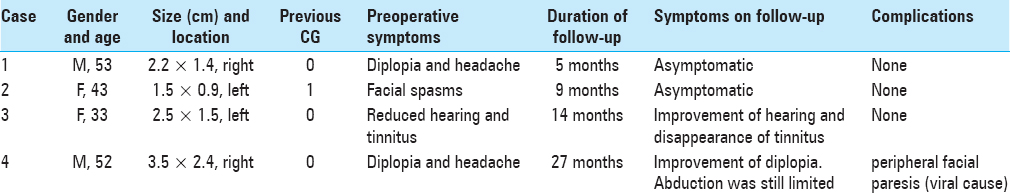
A 53-year-old male patient, with the diagnosis of CG since 2003 with slow progression, presented with diplopia and headache for a couple of weeks. MRI showed a 2.2 × 1.4 cm lesion in the transversal plane above the apex of the right os petrosum. The lesion appeared with a high intensity signal on both T1 and T2-weighted imaging. The rim of the lesion showed a low intensity signal on T2-weighted imaging.
Case 2
A 43-year-old female patient, previously operated for CG in 2009, was experiencing facial spasms for 2 months along with an occipital headache. MRI showed a 1.5 × 0.9 cm lesion in the apex of the left os petrosum. The lesion demonstrated a high intensity signal on both T1 and T2-weighted images which was indicative for recurrence.
Case 3
An incidental finding in a 33-year-old female patient revealed a CG in the apex of the left os petrosum. The MRI showed a heterogeneous lesion with a size of 2.5 × 1.5 cm. T1-weighted imaging revealed a bright lesion in the caudal and dorsal parts, but hypointense in the ventral one. The high signal did not disappear on T1 with fat suppression. Distribution was not restricted. The patient suffered from a generalized epileptic seizure of the left hemisphere, which is unrelated to the CG. After treatment, with the use of carbamazepine, no additional seizures occurred.
Case 4
A 52-year-old male patient had been experiencing paresis of the right abducence nerve for 8 months. The MRI showed a 3.5 × 2.4 cm lesion above the apex of the right os petrosum. The lesion showed a high intensity signal on T1-weighted imaging. Even though the cranial part of the lesion also showed a high-intensity signal on T2-weighted imaging, the caudal portion illustrated a low intensity signal [
RESULTS
Diplopia and headaches were the most common presenting symptoms in our population. All four patients underwent surgery via the middle fossa approach. A summary of patient characteristics at presentation and surgical outcome is presented in
DISCUSSION
CG is a rare, benign entity that is most commonly found in the petrous apex.[
The use of a vascularized temporal muscle flap did not seem to be an effective approach in our clinic because we observed muscle atrophy. However, cautious interpretation of results should be applied as information regarding the average period for CG recurrence is scarce, and thus, our follow-up period might also be too short. In addition, the sample size is rather small due to the rarity of the disease and the relatively short inclusion period.
We could not find any information regarding infection rate after drain placement in the literature. However, a superimposed infection of the drain is a possibility. In addition, displacement of the drain can be a possible complication. These complications have not been observed in our case series. Therefore, we will follow-up with our patients for a longer period. Furthermore, to reduce the likelihood of fibrous formation in the drain, we will explore the possibility of designing a three-dimensional printed biocompatible drain between the mastoid air cell and petrous apex.
CONCLUSION
Our approach of using a SAH drain between the petrous apex and mastoid air cell seems to be effective in achieving constant aeration and contributes toward the prevention of recurrence in CG.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Barath K, Huber AM, Stampfli P, Varga Z, Kollias S. Neuroradiology of cholesteatomas. AJNR Am J Neuroradiol. 2011. 32: 221-9
2. Brackmann DE, Toh EH. Surgical management of petrous apex cholesterol granulomas. Otol Neurotol. 2002. 23: 529-33
3. Castillo MP, Samy RN, Isaacson B, Roland PS. Petrous apex cholesterol granuloma aeration: Does it matter?. Otolaryngol Head Neck Surg. 2008. 138: 518-22
4. DiNardo LJ, Pippin GW, Sismanis A. Image-guided endoscopic transsphenoidal drainage of select petrous apex cholesterol granulomas. Otol Neurotol. 2003. 24: 939-41
5. Eisenberg MB, Haddad G, Al-Mefty O. Petrous apex cholesterol granulomas: Evolution and management. J Neurosurg. 1997. 86: 822-9
6. Hoa M, House JW, Linthicum FH, Go JL. Petrous apex cholesterol granuloma: Pictorial review of radiological considerations in diagnosis and surgical histopathology. J Laryngol Otol. 2013. 127: 339-48
7. Jackler RK, Cho M. A new theory to explain the genesis of petrous apex cholesterol granuloma. Otol Neurotol. 2003. 24: 96-106
8. Kusumi M, Fukushima T, Mehta AI, Cunningham CD, Friedman AH, Fujii K. Middle fossa approach for total resection of petrous apex cholesterol granulomas: Use of vascularized galeofascial flap preventing recurrence. Neurosurgery. 2013. 72: 77-86
9. Mehta RP, Cueva RA, Brown JD, Fliss DM, Gil Z, Kassam AB. What's new in skull base medicine and surgery? Skull Base Committee Report. Otolaryngol Head Neck Surg. 2006. 135: 620-30
10. Royer MC, Pensak ML. Cholesterol granulomas. Curr Opin Otolaryngol Head Neck Surg. 2007. 15: 319-22
11. Samadian M, Akbari Dilmaghani N, Ahmady Roozbahany N, Farzin N, Bahadoram M. Endoscopic Transnasal Approach for Cholesterol Granuloma of the Petrous Apex. Case Rep Neurol Med 2015. 2015. p.
12. Sweeney AD, Osetinsky LM, Carlson ML, Valenzuela CV, Frisch CD, Netterville JL. The Natural History and Management of Petrous Apex Cholesterol Granulomas. Otol Neurotol. 2015. 36: 1714-9
13. Terao T, Onoue H, Hashimoto T, Ishibashi T, Kogure T, Abe T. Cholesterol granuloma in the petrous apex: Case report and review. Acta Neurochir (Wien). 2001. 143: 947-52