- Department of Neurosurgery, Konyang University Hospital, Konyang University College of Medicine, Daejeon, Republic of Korea
- Department of Neurosurgery, College of Medicine, Seoul St. Mary's Hospital, The Catholic University of Korea, Seoul, Republic of Korea
Correspondence Address:
Yong Sam Shin
Department of Neurosurgery, College of Medicine, Seoul St. Mary's Hospital, The Catholic University of Korea, Seoul, Republic of Korea
DOI:10.4103/2152-7806.183497
Copyright: © 2016 Surgical Neurology International This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Song J, Shin YS. Diabetes may affect intracranial aneurysm stabilization in older patients: Analysis based on intraoperative findings. Surg Neurol Int 03-Jun-2016;7:
How to cite this URL: Song J, Shin YS. Diabetes may affect intracranial aneurysm stabilization in older patients: Analysis based on intraoperative findings. Surg Neurol Int 03-Jun-2016;7:. Available from: http://surgicalneurologyint.com/surgicalint_articles/diabetes-may-affect-intracranial-aneurysm-stabilization-in-older-patients-analysis-based-on-intraoperative-findings/
Abstract
Background:Only a small proportion of aneurysms progress to rupture. Previous studies have focused on predicting the rupture risk of intracranial aneurysms. Atherosclerotic aneurysm wall appears resistant to rupture. The purpose of this study was to evaluate clinical and morphological factors affecting atherosclerosis of an aneurysm and identify the parameters that predict aneurysm stabilization.
Methods:We conducted a retrospective analysis of 253 consecutive patients with 291 unruptured aneurysms who underwent clipping surgery in a single institution between January 2012 and October 2013. Aneurysms were categorized based on intraoperative video findings and assessed morphologic and demographic data. Aneurysms which had the atherosclerotic wall without any super thin and transparent portion were defined as stabilized group and the others as a not-stabilized group.
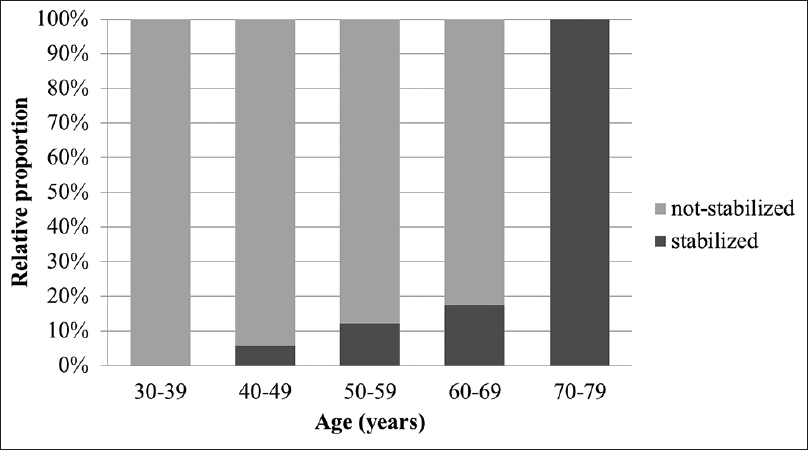
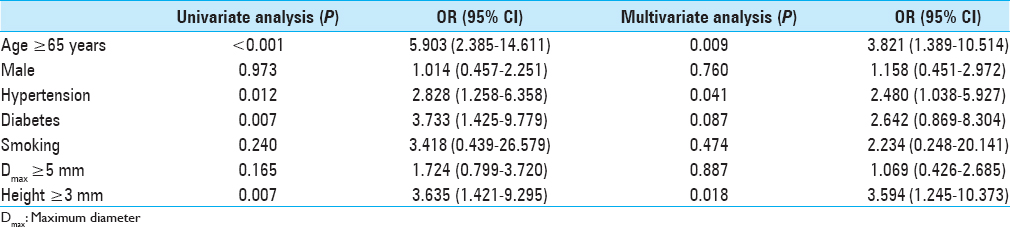
Results:Of the 207 aneurysms, 176 (85.0%) were assigned to the not-stabilized group and 31 (15.0%) to the stabilized group. The relative proportion of stabilized aneurysms increased significantly as the age increased (P P P = 0.012), diabetes (P = 0.007), and height ≥3 mm (P = 0.007) were correlated with stabilized aneurysms. Multivariate logistic analysis showed that age ≥65 years (P = 0.009) and hypertension (P = 0.041) were strongly correlated with stable aneurysms. In older patients (≥65 years of age), multivariate logistic regression revealed that only diabetes was associated with stabilized aneurysms (P = 0.027).
Conclusions:In patients ≥65 years of age, diabetes mellitus may highly predict the stabilized aneurysms. These results provide useful information in determining treatment and follow-up strategies, especially in older patients.
Keywords: Atherosclerosis, intracranial aneurysm, rupture risk, stabilization
INTRODUCTION
Intracranial aneurysms (IAs) occur in approximately 3% of the population,[
The presence of atherosclerotic plaque in some IAs and similar histological and biochemical features of aneurysms and atherosclerotic lesions suggest that atherosclerosis may be a mechanism in the pathogenesis of IAs. Furthermore, atherosclerosis and IA share risk factors, such as hypertension and smoking. Aneurysms may rupture when the thinned wall can no longer withstand the tension.[
The natural history of IA consists of three phases: Initiation, growth, and either stabilization or rupture with only a small minority of aneurysms progressing to rupture.[
MATERIALS AND METHODS
Patients
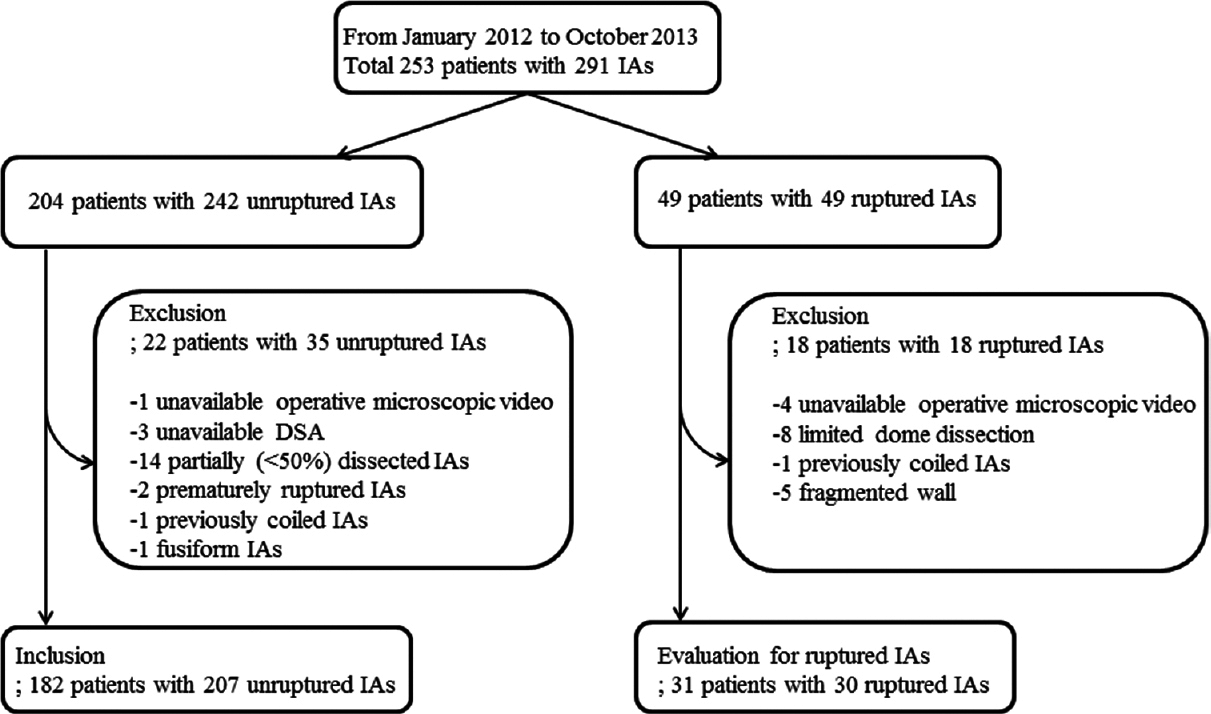
This study was conducted in accordance and with the approval of the Ethics Review Board of our hospital. All patients who underwent microsurgical clipping for ruptured or unruptured IAs between January 2012 and October 2013 in our department were identified using a prospectively collected database. Two hundred and fifty-three consecutive patients with 291 IAs were identified (204 patients with 242 unruptured IAs and 49 patients with 49 ruptured IAs) [
Intraoperative video data
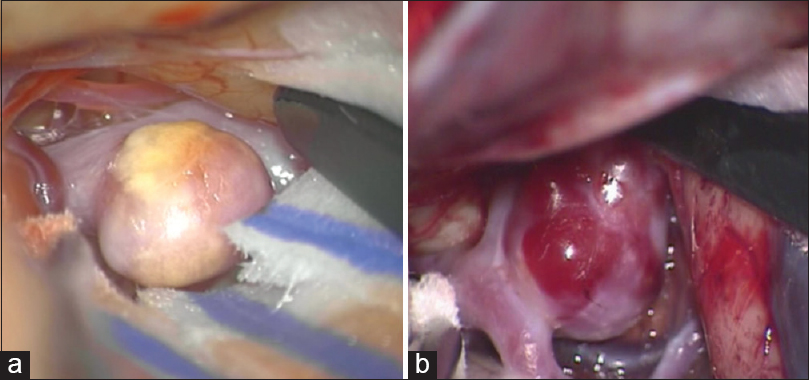
All intraoperative images were captured through a Pentero 900 or Pentero surgical microscope video (Carl Zeiss, Oberkochen, Germany) at 1920 × 1080 or 720 × 480 pixels during aneurysm clipping procedures. Aneurysms were assessed for their wall thickness based on color translucence and the presence of atherosclerosis and divided into two groups. Aneurysms with atherosclerotic wall without any transparent and super thin portion were defined as stabilized aneurysm range and the others as not-stabilized range [
Figure 2
Intraoperative microscopic image of stabilized and not-stabilized aneurysm. (a) Stabilized aneurysm that was with thick atherosclerotic wall without any transparent and super thin portion. (b) Not-stabilized aneurysm that was with transparent and super thin portion and without atherosclerotic wall
We reviewed microscopic videos of 49 consecutive ruptured aneurysm patients (41 females and eight males, mean age 57.6 years, range: 35–83 years) who underwent clipping surgery in our institution for the same period to evaluate whether aneurysms with atherosclerotic walls were stabilized. Because blood obscured the aneurysm dome, identification of the wall appearance was difficult. Of the 49 aneurysms, 18 aneurysms could not be evaluated because of limited dome dissection, absence of microscopic video, coiled aneurysm, or completely fragmented wall.
Demographic, angiographic, and intraoperative video data
Demographic data were reviewed from medical records. In this study, we used the following factors such as patient age, gender, current smoking habit, family history of stroke (both ischemic and hemorrhagic), hypertension (systolic blood pressure >140 mmHg or diastolic blood pressure >90 mmHg) or current treatment status, and diabetes mellitus (DM, hemoglobin A1c ≥6.5) or current treatment status. Aneurysm size was defined as previously described.[
Statistics
The continuous data were expressed as a mean ± standard deviation. Chi-square test or Fishers exact test was used to compare medical comorbidities and dichotomized morphological parameters between stabilized and not-stabilized groups. An unpaired t-test was used for continuous variable statistical analysis. To identify the independent parameters that correlated significantly with the stabilization, univariate and multivariate logistic regression analyses were separately performed for all aneurysms. The odds ratio (OR) and 95% confidence interval (CI) were calculated. A P < 0.05 was considered significant. Statistical analysis was performed using SPSS (SPSS Inc., Chicago, IL, USA).
RESULTS
Intraoperative findings of ruptured aneurysm
Thirty-one ruptured aneurysms (five males and 26 females; mean age 57.35 years, range: 35–79 years) were evaluated for their wall morphology. Of the 31 evaluated aneurysms, only one patient, a 75-year-old female, was suspected of a stabilized aneurysm.
Demographics and morphologies of all aneurysms
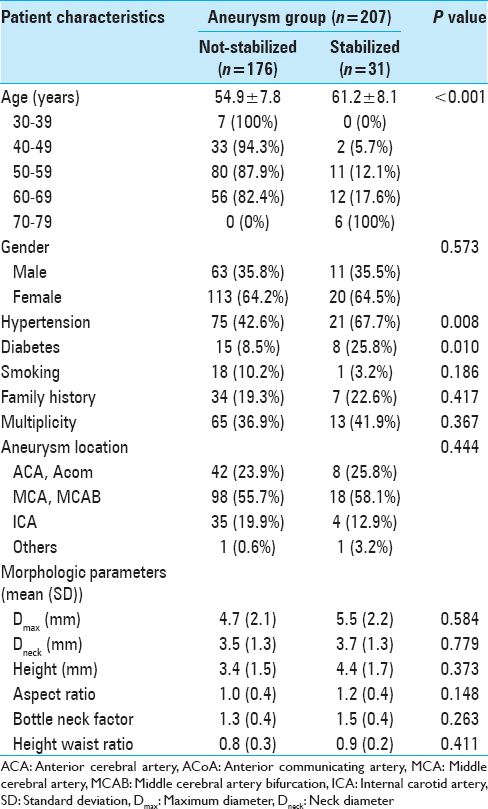
Of the 207 aneurysms, 176 (85.0%) were assigned to the not-stabilized group and 31 (15.0%) to the stabilized group.
To evaluate the predictive factors of stabilized aneurysms, statistical analyses were performed with dichotomized values [
Older patients (≥65 years of age)
There were 26 patients (eight males and 18 females) older than 65 years of age and 11 (42.3%) were in the stabilized group. Medical comorbidities and aneurysm size were analyzed. There were no smokers in this group; thus, we could not obtain a value for smoking with multivariate analysis. Univariate logistic regression analysis showed that DM was the only significant predictor of stabilized aneurysms (P = 0.019), and it remained after multivariate logistic regression analysis (P = 0.027; OR, 29.435; 95% CI, 1.471–588.999).
DISCUSSION
When IAs detected incidentally, physicians decide whether to treat or not by weighing up the risk of treatment against the rupture risk. The decision is often not straightforward because the complication associated with the treatment of unruptured aneurysms are up to 5%, and knowledge regarding the natural history of IAs is limited. To identify the factors which affect aneurysm rupture or stabilization if possible can help deciding treatment and follow-up strategy. With the hypothesis that aneurysms with atherosclerotic wall are stabilized, we found that the relative proportion of stabilized aneurysms significantly increased as age increased and age showed a positive relationship with stabilized aneurysms. In older patients (≥65 years of age), diabetes remained the only independent factor predicting stabilized aneurysms.
Aneurysm wall thickness and rupture risk
Several studies reported that thin portion of aneurysm dome correlated with the point of rupture and clearly defined foci of translucency suggest focal weakness by influencing local stiffness and predisposes these regions to rupture.[
Patient age and aneurysm size
Despite intensive research, the pathogenesis of IA remains unclear but can be related to the mechanisms of atherosclerosis.[
Diabetes mellitus and hypertension as risk factors
Atherosclerosis and aneurysm formation share similar risk factors including smoking and hypertension.[
DM plays an important role in the pathogenesis of atherosclerosis but has not been sufficiently investigated to suggest its role in aneurysm formation or rupture. DM induces vascular endothelial damage and dysfunction, decreases cerebral tight junction protein expression, and[
Hypertension is a well-known risk factor for atherosclerosis and is considered a risk factor for aneurysm formation and rupture.[
Advance in knowledge and clinical implications
To the best of our knowledge, this is the first report investigating clinical and morphological characteristics of atherosclerotic lesions in IAs and the first attempt to identify factors associated with aneurysm stabilization. Age (P = 0.002; OR, 1.105) showed a positive trend with atherosclerotic aneurysm walls and DM (P = 0.027; OR, 29.435) was the only factor predicting stabilized aneurysms in older patients.
The positive association of age and atherosclerotic aneurysm walls suggest the aneurysm may progress with atherosclerotic changes over time and may help to predict the natural history of the aneurysm. The suggested parameters for predicting aneurysm stabilization can help in determining treatment strategy when an unruptured aneurysm is found incidentally. First, the parameters can help in deciding whether to treat or not, especially in elderly patients who have a short life expectancy and usually a higher morbidity rate under general anesthesia. Second, the parameters can help estimate the potential risk of treatment because atherosclerotic aneurysms usually have a higher risk of intraoperative ischemia[
Limitations
The first limitation of this study is the uncertainty whether aneurysms with atherosclerotic walls are stabilized in terms of rupture because the natural history or the rupture mechanism of the aneurysm is unknown. Moreover, there are no large and prospective data showing aneurysm wall morphology and rupture risk. Instead, there are studies that thin portion of aneurysm dome correlated with the point of rupture.[
CONCLUSIONS
Based on the hypothesis that aneurysms with atherosclerotic walls are stabilized aneurysms, we revealed the relative proportion of stabilized aneurysms increased significantly as age increased. In addition, in older patients, DM was the only factor predicting aneurysm stabilization. These results provide useful information for determining treatment and follow-up strategies, especially in elderly patients.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Adams HP, Putman SF, Kassell NF, Torner JC. Prevalence of diabetes mellitus among patients with subarachnoid hemorrhage. Arch Neurol. 1984. 41: 1033-5
2. Bacigaluppi S, Piccinelli M, Antiga L, Veneziani A, Passerini T, Rampini P. Factors affecting formation and rupture of intracranial saccular aneurysms. Neurosurg Rev. 2014. 37: 1-14
3. Baharoglu MI, Lauric A, Gao BL, Malek AM. Identification of a dichotomy in morphological predictors of rupture status between sidewall- and bifurcation-type intracranial aneurysms. J Neurosurg. 2012. 116: 871-81
4. Bekelis K, Missios S, MacKenzie TA, Desai A, Fischer A, Labropoulos N. Predicting inpatient complications from cerebral aneurysm clipping: The Nationwide Inpatient Sample 2005-2009. J Neurosurg. 2014. 120: 591-8
5. Bijlenga P, Ebeling C, Jaegersberg M, Summers P, Rogers A, Waterworth A. Risk of rupture of small anterior communicating artery aneurysms is similar to posterior circulation aneurysms. Stroke. 2013. 44: 3018-26
6. Brisman JL, Song JK, Newell DW. Cerebral aneurysms. N Engl J Med. 2006. 355: 928-39
7. Cebral JR, Mut F, Weir J, Putman CM. Association of hemodynamic characteristics and cerebral aneurysm rupture. AJNR Am J Neuroradiol. 2011. 32: 264-70
8. Crompton MR. Mechanism of growth and rupture in cerebral berry aneurysms. Br Med J. 1966. 1: 1138-42
9. Damsgaard EM, Frøland A, Green A, Hauge M. An alternative sampling approach to the study of diabetes prevalence. Scand J Soc Med. 1984. 12: 115-20
10. D’Andrea V, Cantisani V, Catania A, Todini A, Stio F, Di Matteo FM. Angiomegaly and arterial aneurysms. G Chir. 2010. 31: 429-32
11. Dhar S, Tremmel M, Mocco J, Kim M, Yamamoto J, Siddiqui AH. Morphology parameters for intracranial aneurysm rupture risk assessment. Neurosurgery. 2008. 63: 185-96
12. Feigin VL, Rinkel GJ, Lawes CM, Algra A, Bennett DA, van Gijn J. Risk factors for subarachnoid hemorrhage: An updated systematic review of epidemiological studies. Stroke. 2005. 36: 2773-80
13. Frösen J, Piippo A, Paetau A, Kangasniemi M, Niemelä M, Hernesniemi J. Remodeling of saccular cerebral artery aneurysm wall is associated with rupture: Histological analysis of 24 unruptured and 42 ruptured cases. Stroke. 2004. 35: 2287-93
14. Gabriel RA, Kim H, Sidney S, McCulloch CE, Singh V, Johnston SC. Ten-year detection rate of brain arteriovenous malformations in a large, multiethnic, defined population. Stroke. 2010. 41: 21-6
15. Greenhalgh RM, Laing S, Taylor GW. Risk factors in carotid artery stenosis and intracranial aneurysms. J Cardiovasc Surg (Torino). 1980. 21: 559-67
16. Greving JP, Wermer MJ, Brown RD, Morita A, Juvela S, Yonekura M. Development of the PHASES score for prediction of risk of rupture of intracranial aneurysms: A pooled analysis of six prospective cohort studies. Lancet Neurol. 2014. 13: 59-66
17. Hashimoto N, Handa H, Hazama F. Experimentally induced cerebral aneurysms in rats. Surg Neurol. 1978. 10: 3-8
18. Hashimoto N, Handa H, Hazama F. Experimentally induced cerebral aneurysms in rats: Part II. Surg Neurol. 1979. 11: 243-6
19. Hashimoto T, Meng H, Young WL. Intracranial aneurysms: Links among inflammation, hemodynamics and vascular remodeling. Neurol Res. 2006. 28: 372-80
20. Hoh BL, Sistrom CL, Firment CS, Fautheree GL, Velat GJ, Whiting JH. Bottleneck factor and height-width ratio: Association with ruptured aneurysms in patients with multiple cerebral aneurysms. Neurosurgery. 2007. 61: 716-22
21. Inagawa T. Risk factors for the formation and rupture of intracranial saccular aneurysms in Shimane, Japan. World Neurosurg. 2010. 73: 155-64
22. Juvela S, Porras M, Heiskanen O. Natural history of unruptured intracranial aneurysms: A long-term follow-up study. J Neurosurg. 1993. 79: 174-82
23. Kashiwazaki D, Kuroda S. Size ratio can highly predict rupture risk in intracranial small (<5 mm) aneurysms. Stroke. 2013. 44: 2169-73
24. Kataoka K, Taneda M, Asai T, Kinoshita A, Ito M, Kuroda R. Structural fragility and inflammatory response of ruptured cerebral aneurysms. A comparative study between ruptured and unruptured cerebral aneurysms. Stroke. 1999. 30: 1396-401
25. Killer-Oberpfalzer M, Aichholzer M, Weis S, Richling B, Jones R, Virmani R. Histological analysis of clipped human intracranial aneurysms and parent arteries with short-term follow-up. Cardiovasc Pathol. 2012. 21: 299-306
26. Kosierkiewicz TA, Factor SM, Dickson DW. Immunocytochemical studies of atherosclerotic lesions of cerebral berry aneurysms. J Neuropathol Exp Neurol. 1994. 53: 399-406
27. Lindgren AE, Kurki MI, Riihinen A, Koivisto T, Ronkainen A, Rinne J. Type 2 diabetes and risk of rupture of saccular intracranial aneurysm in eastern Finland. Diabetes Care. 2013. 36: 2020-6
28. Loumiotis I, Wagenbach A, Brown RD, Lanzino G. Small (< 10-mm) incidentally found intracranial aneurysms, Part 1: Reasons for detection, demographics, location, and risk factors in 212 consecutive patients. Neurosurg Focus. 2011. 31: E3-
29. Maltete D, Bellien J, Cabrejo L, Iacob M, Proust F, Mihout B. Hypertrophic remodeling and increased arterial stiffness in patients with intracranial aneurysms. Atherosclerosis. 2010. 211: 486-91
30. Morimoto M, Miyamoto S, Mizoguchi A, Kume N, Kita T, Hashimoto N. Mouse model of cerebral aneurysm: Experimental induction by renal hypertension and local hemodynamic changes. Stroke. 2002. 33: 1911-5
31. Muller M, van den Beld AW, Bots ML, Grobbee DE, Lamberts SW, van der Schouw YT. Endogenous sex hormones and progression of carotid atherosclerosis in elderly men. Circulation. 2004. 109: 2074-9
32. Nagamine Y. Natural history and management of asymptomatic unruptured cerebral aneurysms. Rinsho Shinkeigaku. 2004. 44: 763-6
33. Raghavan ML, Ma B, Harbaugh RE. Quantified aneurysm shape and rupture risk. J Neurosurg. 2005. 102: 355-62
34. Reynolds MR, Willie JT, Zipfel GJ, Dacey RG. Sexual intercourse and cerebral aneurysmal rupture: Potential mechanisms and precipitants. J Neurosurg. 2011. 114: 969-77
35. Rinkel GJ, Djibuti M, Algra A, van Gijn J. Prevalence and risk of rupture of intracranial aneurysms: A systematic review. Stroke. 1998. 29: 251-6
36. Sato A, Watanabe S, Okubo S, Toi T, Doi T, Nakano H. The therapeutic importance of home blood pressure assessment and combination antihypertensive therapy for achieving target blood pressure control: Ibaraki hypertension assessment trial. Hypertens Res. 2010. 33: 1264-71
37. Song J, Park JE, Kim HR, Shin YS. Observation of cerebral aneurysm wall thickness using intraoperative microscopy: Clinical and morphological analysis of translucent aneurysm. Neurol Sci. 2015. 36: 907-12
38. Starke RM, Chalouhi N, Ding D, Raper DM, Mckisic MS, Owens GK. Vascular smooth muscle cells in cerebral aneurysm pathogenesis. Transl Stroke Res. 2014. 5: 338-46
39. Tada Y, Wada K, Shimada K, Makino H, Liang EI, Murakami S. Roles of hypertension in the rupture of intracranial aneurysms. Stroke. 2014. 45: 579-86
40. Tamura T, Jamous MA, Kitazato KT, Yagi K, Tada Y, Uno M. Endothelial damage due to impaired nitric oxide bioavailability triggers cerebral aneurysm formation in female rats. J Hypertens. 2009. 27: 1284-92
41. Morita A, Kirino T, Hashi K, Aoki N, Fukuhara S. The natural course of unruptured cerebral aneurysms in a Japanese cohort. N Engl J Med. 2012. 366: 2474-82
42. Vlak MH, Algra A, Brandenburg R, Rinkel GJ. Prevalence of unruptured intracranial aneurysms, with emphasis on sex, age, comorbidity, country, and time period: A systematic review and meta-analysis. Lancet Neurol. 2011. 10: 626-36
43. Wiebers DO, Whisnant JP, O’Fallon WM. The natural history of unruptured intracranial aneurysms. N Engl J Med. 1981. 304: 696-8
44. Wiebers DO, Whisnant JP, Sundt TM, O’Fallon WM. The significance of unruptured intracranial saccular aneurysms. J Neurosurg. 1987. 66: 23-9
45. Wiebers DO, Whisnant JP. Rupture of an intracranial aneurysm. Surg Neurol. 1990. 33: 157-8
46. Yan T, Chopp M, Ning R, Zacharek A, Roberts C, Chen J. Intracranial aneurysm formation in type-one diabetes rats. PLoS One. 2013. 8: e67949-
47. Ye X, Chopp M, Cui X, Zacharek A, Cui Y, Yan T. Niaspan enhances vascular remodeling after stroke in type 1 diabetic rats. Exp Neurol. 2011. 232: 299-308