- Department of Neurosurgery, Cedar Stem Cell Institute, Town Street, Columbus, Ohio, USA
Correspondence Address:
J. A. Shehadi
Department of Neurosurgery, Cedar Stem Cell Institute, Town Street, Columbus, Ohio, USA
DOI:10.4103/sni.sni_155_17
Copyright: © 2017 Surgical Neurology International This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: J. A. Shehadi, S. M. Elzein. Review of commercially available demineralized bone matrix products for spinal fusions: A selection paradigm. 22-Aug-2017;8:203
How to cite this URL: J. A. Shehadi, S. M. Elzein. Review of commercially available demineralized bone matrix products for spinal fusions: A selection paradigm. 22-Aug-2017;8:203. Available from: http://surgicalneurologyint.com/surgicalint-articles/review-of-commercially-available-demineralized-bone-matrix-products-for-spinal-fusions-a-selection-paradigm/
Abstract
Background:Spinal fusions are commonly performed in the US each year for various spinal pathologies. There are multiple commercially available graft material options for these procedures, including an abundance of demineralized bone matrix (DBM) products.
Methods:This study reviews, clearly organizes, and puts forth meaningful information on select biological and physical properties of several commercially available DBM products. In addition, we provide an alternative classification method of DBM products by carrier.
Results:This review takes a closer look at the commercial and distributor practices of these products and companies in order to increase transparency between the consumer and source companies.
Conclusions:We propose a novel patient-centered approach to DBM product selection. This requires prioritizing patient safety, product effectiveness, and product transparency. This review offers a practical paradigm to facilitate informed product choice for surgeons and hospital systems alike.
Keywords: Allograft bone, demineralized bone matrix, DBM, iliac crest bone graft
INTRODUCTION
Between 1998 and 2008, the annual number of spinal fusion discharges in the United States increased 137%, causing a 7.9-fold increase in the national bill for spinal fusion.[
Iliac crest bone graft (ICBG) has been the historical “gold-standard” source of autograft material used to promote spinal fusion. The advantages of ICBG and other local autografts are their osteoinductive (OI), osteoconductive, and osteogenic properties (e.g., no risk of rejection or disease transmission). Disadvantages include (1) a second surgical site, (2) graft site morbidity (often exaggerated), and (3) longer operation times. Furthermore, ICBG and other local autografts may not provide sufficient bone material for larger fusion areas, or there may be other host factors that contraindicate its use including active infection, tumor, or severe osteoporosis.
Therefore, surgeons have long turned away from ICBG to allogeneic or synthetic sources of bone graft material, including freeze-dried allograft, fresh-frozen allograft, cancellous chips, ceramics, bone morphogenetic proteins (BMPs), demineralized bone matrix (DBM), and other permutations.[
The turn away from ICBG has facilitated the rise of many commercially available DBM products. There are few, if any, well-established algorithms or systematic approaches for surgeons and hospitals to decide which DBM products to utilize. This report discusses product safety, transparency and accurate labeling, biological properties, and physical properties while proposing a model paradigm that will facilitate effective DBM product selection.
MATERIALS AND METHODS
Ideal biological properties of demineralized bone matrix
The ideal DBM bone graft material (1) resists disease transmission or immune-mediated rejection, (2) incorporates completely into resident tissue, (3) promotes surface-level bone growth (termed osteoconductivity), (4) promotes bone-forming cells (osteoinductivity), and (5) fosters controlled osteogenesis, so as not to produce superfluous bone.[
Ideal physical properties of demineralized bone matrix
A DBM graft material with ideal physical properties (1) increases total mass of the graft (graft extender), (2) serves as a scaffold with good mechanical strength, and (3) possesses favorable handling characteristics (e.g., pliability for manipulation into any size/shape to prevent migration).
Harvesting and processing
There is significant variability in how DBM products are extracted, processed, and packaged. In general, DBM is typically extracted from cortical allograft bone, demineralized via acid extraction, sterilized, and combined with a carrier to prevent migration. Each DBM product has its advantages and disadvantages leading to variable fusion rates.[
“White Boxing”
The term “white-boxing” is used describe the relative lack of information included with the different DBM products. Missing details include whether the product comes from a living or deceased donor, domestic or international source, and/or the bone composition of the product. While the Food and Drug Administration (FDA) uses the 510(k)
Variability of demineralized bone matrix products
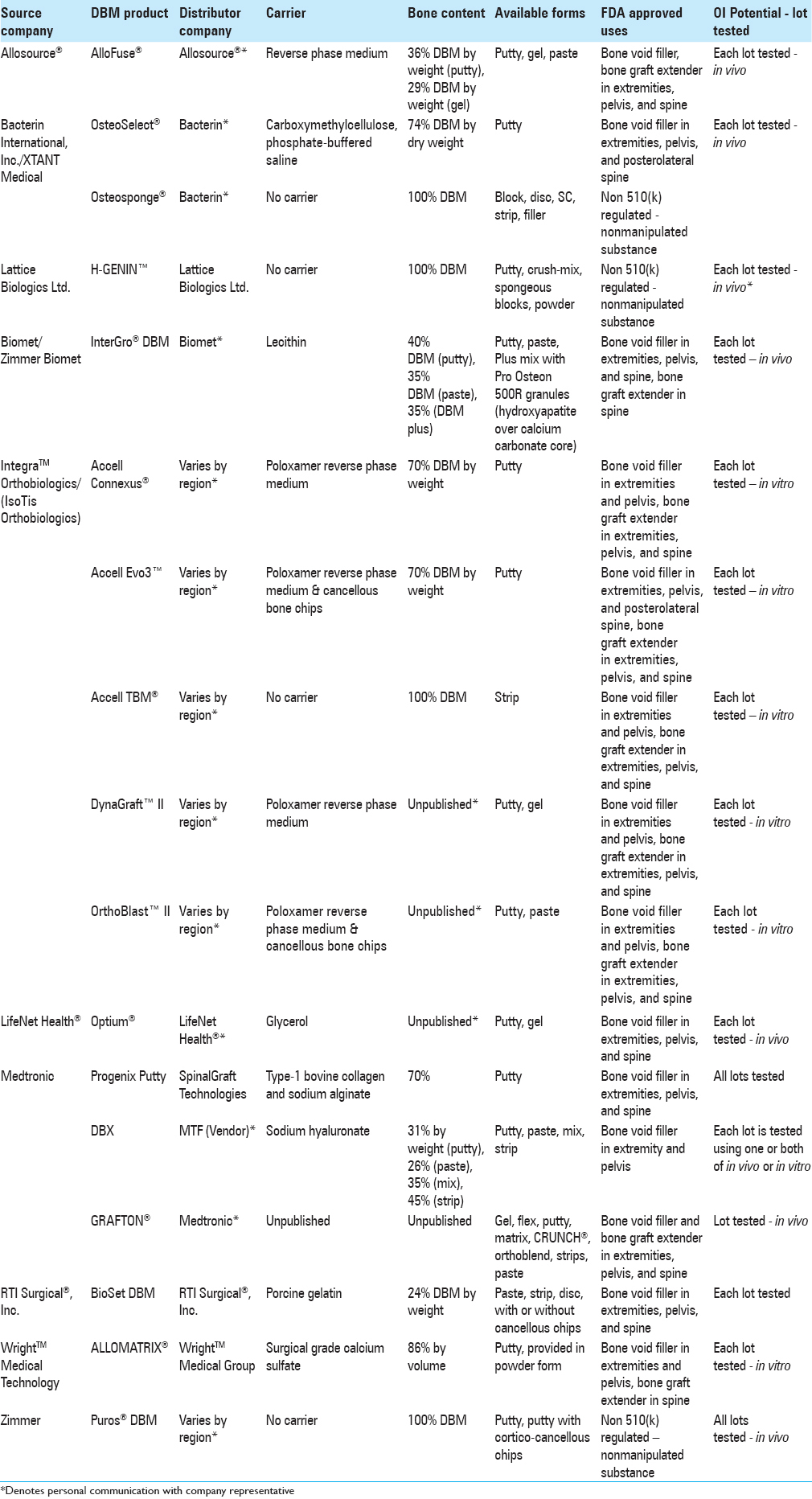
Variability of DBM products has critical and lasting consequences for both clinicians and their patients. Here, we review the distributive methods, biological properties and quality control of several well-known DBM products in the interest of full disclosure and transparency. The authors synthesize and display information on “Distributor Company” and “Bone Content” of 33 DBM products. Note that distributor companies vary by region.
Flyers
Materials on each product were obtained either through the source company's website or by contacting a company representative directly. Information not available in published documents are cited as personal communication or unpublished [Tables
RESULTS
Summary of demineralized bone matrix products
There were 17 DBM products evaluated from ten source companies [
DBM products come in a variety of forms, including sponges, strips, injectable putty, paste, and paste infused with chips. These various forms affect the products’ ability to serve as graft extenders, enhancers, or substitutes.
Demineralized bone matrix products organized by carrier
Thirty-three DBM products were organized by carrier substance [
Consideration of non-inflammatory properties of demineralized bone matrix
While the non-inflammatory qualities of DBM have been considered a beneficial characteristic of carriers, the precise role of inflammation in spinal fusion remains controversial. Inflammation plays a key role in recruiting osteoblasts to produce new bone, promote fusion, facilitate early healing, and encourage angiogenesis. Therefore, carriers with strong anti-inflammatory characteristics may not generate an optimal postoperative healing environment. On the other hand, excessive inflammation at the fusion site may lead to patient complications such as pain and stiffness. It is the surgeon-authors’ opinion that some inflammation is useful to facilitate bone fusion.
Impact of carriers for demineralized bone matrix products
Each carrier substance imparts its own unique properties onto its respective combined DBM product. Examples of commercially utilized carriers include calcium sulphate (Wright's Allomatrix®), sodium hyaluronate (Medtronic's DBX®), and porcine collagen (Medtronics’ Osteofil®) [
Similarity in demineralized bone matrix product nomenclature
White-boxed DBM products often share similar names leading to confusion. The names of the original bone source company, as well as the names of distributors and end products are strikingly similar, e.g., the AlloSource company produces AlloFuse® DBM, not to be confused with Wright Medical Technology's AlloMatrix® DBM [
DISCUSSION
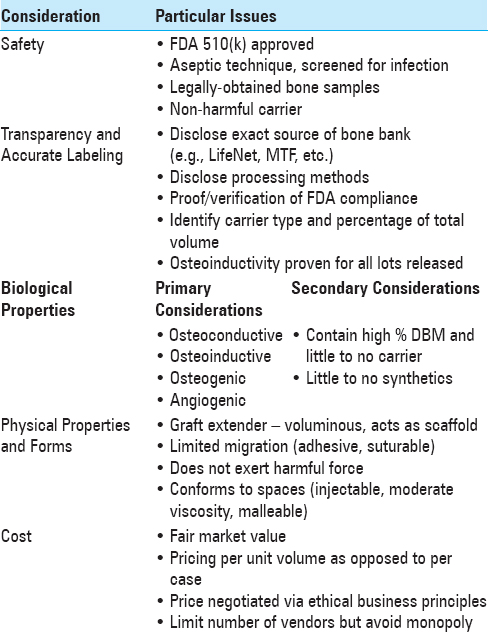
The often confusing diversity of commercially available DBM products presents a problem to clinicians and hospital systems whose goal is to select the most efficient, cost-effective, and proven successful products for their patients. As mentioned before, variability of DBM products has important and lasting consequences for both clinician and patient. Of paramount importance in choosing a DBM product and successful clinical implementation are the following aspects:[
Donor selection (age, gender, drug use/abuse habits, HIV status, cancer history) Composition of donor tissue (BMP, other protein content, particle size, and biologics) Freeze-dried packaging methods Lot sampling for osteoinductive index Transparency in labeling of such information.
There is significant variability with which present-day DBM products are extracted, processed, and packaged. Demineralized bone matrices are often developed through extraction of cortical allograft bone that subsequently undergoes demineralization via acid extraction, sterilization, and combination with a carrier to prevent migration. However, depending on the preparation methods utilized, each DBM product offers assorted advantages and disadvantages leading to variable fusion rates. These factors not only establish variability between products but may also contribute to intravariability of several characteristics within the same batch of a given DBM product.
Despite the extreme variability in the number and types of DBM products complicating product selection, their efficacy has been proven in treating multilevel cervical disc disease[
One of the objectives of this paper is to elucidate and help unravel the diverse slate of DBM products and the complex process of selecting one for use in patients. Synthesizing the available information and without favoring any one company or distributor, the surgeon-author can make several suggestions, without specific endorsements. These suggestions are based on published information provided, keeping in mind several companies have not fully disclosed the information on exact content and percentage by weight of DBM. Thus, in addition to cost concerns (which vary), and transparency of product content, a hospital and surgeon should be well served with the following abbreviated list of DBM products.
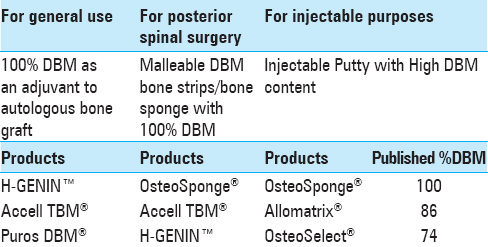
For general routine use of DBM as an adjuvant to autologous bone graft for spinal fusions, the surgeon-author suggests a pure 100% DBM paste without carrier such as H-GENIN™, Accell TBM®, or Puros DBM® [
In addition, for posterior spinal surgery, the surgeon-author recommends a DBM bone strip, which is soft and porous and offers more immediate support and scaffolding. The strip is especially useful if soaked in an autologous Bone Marrow Aspirate Concentration (BMAC). Among those listed in
On occasion, there is a need to inject DBM into a bone defect, e.g., when caused by the removal of a Pedicle screw. For this purpose, the surgeon-author proposes using a DBM with a carrier, making it amenable to placement into a syringe and viscous enough to be injected through a syringe and applicator (such as OsteoSponge® syringe, Allomatrix® injectable putty, and OsteoSelect®). See
It is difficult to meaningfully select DBM products if the information is not posted on packaging material. Surgeons and hospital systems must “raise the bar” when selecting DBM products. It is the responsibility of companies and the FDA to make available the origin, processing, storage parameters, and the final DBM content of these products. Increased transparency through better labeling will lead to greater knowledge and patient safety. It is also acceptable to have fewer, but better DBM products.
FUTURE DIRECTIONS
In the future, DBM products may be gradually replaced or enhanced by more effective treatment modalities such as autologous stem cells from bone marrow aspirates. The industry has already begun to develop new products promoting greater angiogenesis (adding vascular endothelial growth factor (VEGF) as seen in Bio4®). Other companies propose bone grafts with live stem cells already committed to differentiation into osteocyte lineage (ViviGen® Cellular Bone Matrix). While these newer treatment methods/products are still being developed, there is an immediate need to be equipped to choose the “best” DBM products for our patients.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
The authors acknowledge Liliane Dableh, Ph.D., Bachir Abi Salloum, Ph.D. and Rashmi Nemade, Ph.D. for their assistance in reviewing the manuscript and performing literature searches
References
1. Albrektsson T, Johansson C. Osteoinduction, osteoconduction and osseointegration. Eur Spine J. 2001. 10: S96-101
2. Bae H, Zhao L, Zhu D, Kanim LE, Wang JC, Delamarter RB. Variability across ten production lots of a single demineralized bone matrix product. J Bone Joint Surg Am. 2010. 92: 427-35
3. Bae HW, Zhao L, Kanim LE, Wong P, Delamarter RB, Dawson EG. Intervariability and intravariability of bone morphogenetic proteins in commercially available demineralized bone matrix products. Spine. 2006. 31: 1299-306
4. Last accessed on 2017 Jun 09. Available from: http://www.aatb.org/sites/default/files/BoneGraftSubstituteTable2010.pdf .
5. Campana V, Milano G, Pagano E, Barba M, Cicione C, Salonna G. Bone substitutes in orthopaedic surgery: From basic science to clinical practice. J Mater Sci Mater Med. 2014. 25: 2445-61
6. Demircan MN, Kutlay AM, Colak A, Kaya S, Tekin T, Kibici K. Multilevel cervical fusion without plates, screws or autogenous iliac crest bone graft. J Clin Neurosci. 2007. 14: 723-8
7. Deyo RA. Fusion surgery for lumbar degenerative disc disease: Still more questions than answers. Spine J. 2015. 15: 272-4
8. Epstein NE. Efficacy of different bone volume expanders for augmenting lumbar fusions. Surg Neurol. 2008. 69: 16-9
9. Last accessed 2017 Jun 09. Available from: https://services.myendnoteweb.com/enwservices/fileattachment/downloadAttachment.jsp? fileName=Bone_Graft_Substitutes_Webinar_FINAL_PDF_9_24_14.pdf&userId=U1yRRQrYHK4AAErH91 0&refId=335&fileNamePlain=Bone_Graft_Substitutes_Webinar_FINAL_PDF_9_24_14.pdf .
10. Rajaee SS, Bae HW, Kanim LE, Delamarter RB. Spinal fusion in the United States: Analysis of trends from 1998 to 2008. Spine. 2012. 37: 67-76
11. Tilkeridis K, Touzopoulos P, Ververidis A, Christodoulou S, Kazakos K, Drosos GI. Use of demineralized bone matrix in spinal fusion. World J Orthop. 2014. 5: 30-7
12. Wildemann B, Kadow-Romacker A, Haas NP, Schmidmaier G. Quantification of various growth factors in different demineralized bone matrix preparations. J Biomed Mater Res A. 2007. 81: 437-42