- Department of Neurosurgery, Showa University School of Medicine, Tokyo, Japan
- Department of Neurosurgery, Cerebrovascular Center, Niigata Rosai Hospital, Japan Organization of Occupational Health and Safety, Niigata, Japan
- Department of Neurosurgery, International Neuroscience Institute, Hannover, Germany
Correspondence Address:
Yosuke Sato
Department of Neurosurgery, International Neuroscience Institute, Hannover, Germany
DOI:10.4103/sni.sni_326_17
Copyright: © 2017 Surgical Neurology International This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Yosuke Sato, Madjid Samii. A technique for sequential, progressive clipping for a giant thrombosed distal anterior cerebral artery aneurysm: Technical note. 06-Dec-2017;8:292
How to cite this URL: Yosuke Sato, Madjid Samii. A technique for sequential, progressive clipping for a giant thrombosed distal anterior cerebral artery aneurysm: Technical note. 06-Dec-2017;8:292. Available from: http://surgicalneurologyint.com/surgicalint-articles/a-technique-for-sequential-progressive-clipping-for-a-giant-thrombosed-distal-anterior-cerebral-artery-aneurysm-technical-note/
Abstract
Background:Giant thrombosed aneurysms often present with thickened walls and a hard thrombus, including in the near-neck aneurysmal sac. These usually make it difficult to achieve complete neck clipping with preservation of local branch patency. Here, we demonstrate a simple but safe and effective technique to overcome these problems in a patient with a 6-cm giant thrombosed distal anterior cerebral artery aneurysm.
Case Description:A 77-year-old-man suffered from loss of volitional activity due to the frontal mass effect. The aneurysm was exposed with unilateral paramedian craniotomy and an interhemispheric approach. The clip was applied to the aneurysmal neck but it slipped onto the parent artery, which caused branch artery occlusion. Intra-aneurysmal thrombectomy was immediately performed near the aneurysmal neck with ultrasonic aspiration. The next clip was added along the aneurysm side of the preceding clip, which was then removed. This procedure was repeated twice so that complete neck clipping was achieved while preserving the branch patency. All the residual thrombus and aneurysmal wall were subsequently removed. Postoperatively, there was no additional neurological deficit. The patient's mental function was significantly improved.
Conclusions:We conclude that the sequential, progressive clipping technique is a robust option for successful neck clipping of giant thrombosed aneurysms.
Keywords: Cerebral aneurysm, distal anterior cerebral artery, giant thrombosed aneurysm, neck clipping, thrombectomy
INTRODUCTION
Giant thrombosed cerebral aneurysms are rarely treated by direct clipping because of the thickened wall, wide neck, and rich thrombus within the near-neck sac.[
CASE REPORT
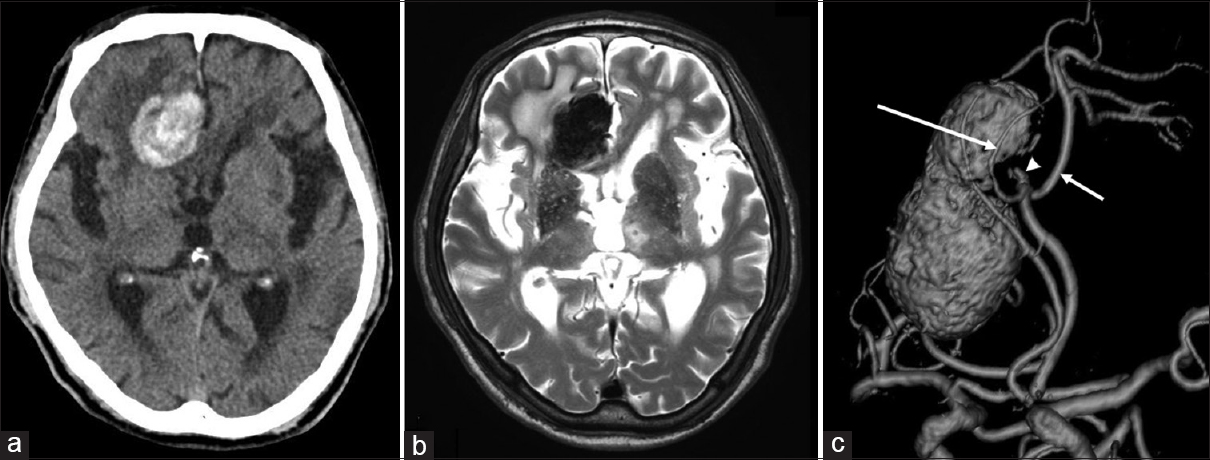
A 77-year-old-man presented with several months of headache and progressive loss of volitional activity. He had normal language function, normal cranial nerves, normal motor/sensory function, and mild gait disturbance. Noncontrast computed tomography (CT) scan and magnetic resonance (MR) imaging of the head revealed a giant thrombosed DACA aneurysm with thickened calcified walls and significant perifocal edema in the right frontal lobe [Figure
Figure 1
(a and b) Preoperative CT scan and MR imaging showing a giant thrombosed DACA aneurysm with calcified wall and perifocal edema in the right frontal lobe. (c) The oblique view of preoperative 3-dimensional MR angiography demonstrating a 6 cm giant thrombosed aneurysm at the left A2-A3 junction. The left PcaA (short arrow) and CmaA (long arrow) originated from the aneurysmal neck. A non-thrombosed area was present within the near-neck aneurysmal sac (arrowhead)
The patient was placed in a supine position with a head elevation of 15°. The head was fixed with Sugita Head Holder. A left-side extended bicoronal skin incision was made. A left frontal craniotomy was performed with an extension of 2 cm across the midline to the right side. The dura mater was incised only on the left side. The arachnoid trabeculations and interdigitating gyri of the interhemispheric fissure were opened sharply by microscissors and bipolar technique. The left A2, PcaA, and CmaA were identified. The aneurysm originated just from the left A2–A3 junction and the major part of the aneurysmal dome invaginated into the right frontal lobe. While almost entire lumen of the aneurysm was thrombosed, the near-neck space was nonthrombosed, which was confirmed by pulsed Doppler. First, a bayonet-shaped clip (7 mm) was applied to the neck but it slipped onto the left A2, which caused the reduction of blood flow both in the left PcaA and CmaA. Immediately after this, the aneurysmal wall was incised and the intra-aneurysmal thrombus was evacuated using the cavitron ultrasonic surgical aspiration (CUSA) until the nonthrombosed near-neck space was exposed. This procedure provided adequate pliability and flexibility of the neck for clipping. The second clip was added parallel and adjacent to the first clip on the aneurysm side, and then the first clip was removed. The blood flow of the left PcaA significantly improved but that of the left CmaA was still poor. In the same manner as mentioned above, a third clip was applied closer to the aneurysm and the second clip was removed. Finally, the blood flow of the left CmaA became sufficient. This sequential, progressive clipping technique following intra-aneurysmal thrombectomy is illustrated in
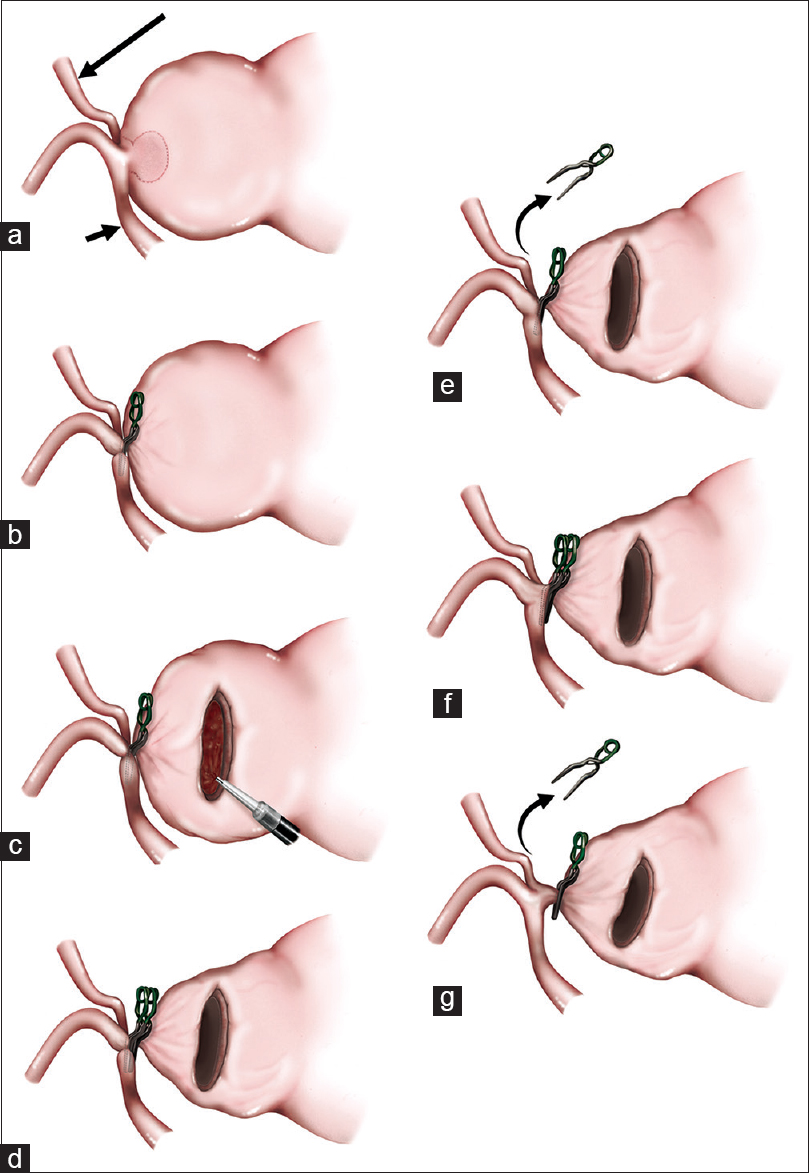
Figure 2
Illustrations of the sequentially shifting clipping technique following intra-aneurysmal thrombectomy. (a) The interhemispheric approach exposed the left A2, PcaA (short arrow), and CmaA (long arrow). The near-neck space (surrounded by a dotted line) was non-thrombosed. (b) A bayonet-shaped clip was applied to the neck, but slipped onto the left A2. (c) The aneurysmal wall was incised and the intra-aneurysmal thrombus was evacuated using CUSA. (d) Once adequate pliability and flexibility of the neck for clipping was obtained, the second clip was added parallel to and adjacent to the aneurysm side of the first clip. (e) The first clip was removed. The blood flow of the left PcaA significantly improved, but that of the left CmaA was still poor. (f) In the same way as in D), the third clip was applied. (g) The second clip was removed. The blood flow of the left CmaA became sufficient. The complete obliteration of the aneurysm was also accomplished
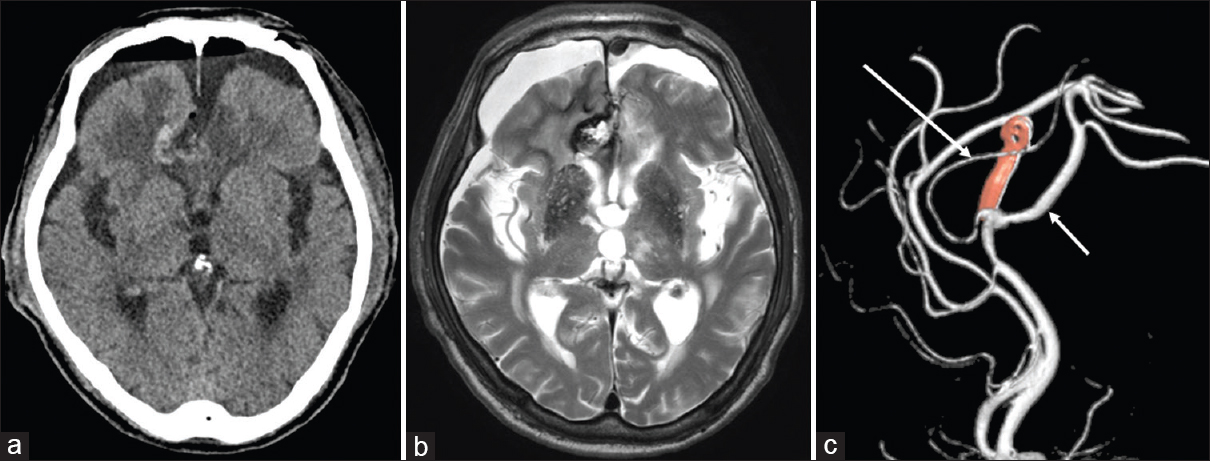
The patient had no additional neurological deficits immediately after surgery. Postoperative CT scan and MR imaging demonstrated no residual aneurysm and resolution of the right frontal edema [Figure
Figure 3
(a, b) Postoperative CT scan and MR imaging showing no residual aneurysm and resolution of the right frontal edema. (c) Postoperative 3-dimentional digital subtraction angiogram demonstrating complete neck clipping of the aneurysm with preservation of all branches (PcaA, short arrow; CmaA, long arrow)
DISCUSSION
Here, we introduce the sequential, progressive clipping technique following intra-aneurysmal thrombectomy as a robust option for surgical treatment of giant thrombosed aneurysms. Performing this technique requires the following: (1) applying at least two clips, finally using a single clip; (2) adequate thrombus evacuation within the near-neck aneurysmal sac by CUSA; and (3) evaluation of hemodynamics on both the local branches and the aneurysmal lumen by pulsed Doppler. The clip is preferably bayonet-shaped. The angle of the bayonet clip allows us to safely occlude the aneurysm with a thickened wall because of the high visibility of the blade tip. The shank of the bayonet clip also makes it easy to arrange two adjacent clips in parallel. The CUSA can evacuate the toughly organized thrombus and its efficacy has been reported for giant thrombosed aneurysms.[
Neck clipping with thrombectomy should be the best strategy for giant thrombosed aneurysm, especially those in which neck is not involved by thrombus.[
A similar technique has been reported by Giannotta as the “stacking-seating” technique using differently shaped and sized clips which are progressively apposed and eventually removed until obtaining exclusion of the aneurysmal sac.[
CONCLUSIONS
For patients with giant thrombosed aneurysms with mass effect, both complete obliteration and resection of aneurysms should be required. Our sequential, progressive clipping technique may be considered as a robust treatment option for such patients. We also hope that this report will be a reminder of what can be done with thoughtful clipping for challenging giant aneurysms in the era of endovascular treatment.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient has given his consent for his images and other clinical information to be reported in the journal. The patient understands that name and initial will not be published and due efforts will be made to conceal identity, but anonymity cannot be guaranteed.
Consent
The patient provided informed consent to the publication of this report.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Bas MB, Guerra N, Valsania V, Boccardo M. Giant intracranial aneurysm of the anterior communicating artery treated by direct surgical approach. Case report. J Neurosurg Sci. 2000. 44: 133-6
2. Cikla U, Uluc K, Baskaya MK. Clip reconstruction of an 8 cm giant internal carotid artery bifurcation aneurysm: Microsurgical technique. Neurosurg Focus. 2015. 38:
3. Farias JP, Trindade AM. Giant distal anterior cerebral artery aneurysm not visualized on angiography: Case report. Surg Neurol. 1997. 48: 348-51
4. Gewirtz RJ, Awad IA. Giant aneurysms of the proximal anterior cerebral artery: Report of three cases. Neurosurgery. 1993. 33: 120-4
5. Giannotta SL. Ophthalmic segment aneurysm surgery. Neurosurgery. 2002. 50: 558-62
6. Hayashi M, Kobayashi H, Kawano H, Handa Y, Kabuto M. Giant aneurysm of an azygous anterior cerebral artery: Report of two cases and review of the literature. Neurosurgery. 1985. 17: 341-4
7. Iihara K, Maruyama D, Fukuda K, Nakajima N, Kataoka H. Tasuki clipping combined with high flow bypass for ruptured, previously coiled, partially thrombosed giant aneurysm at the carotid bifurcation. Neurol Med Chir (Tokyo). 2013. 53: 185-9
8. Kanemoto Y, Tanaka Y, Nonaka M, Hironaka Y. Giant aneurysm of the azygos anterior cerebral artery: Case report. Neurol Med Chir (Tokyo). 2000. 40: 472-5
9. Lawton MT, Spetzler RF. Surgical management of giant intracranial aneurysms: Experience with 171 patients. Clin Neurosurg. 1995. 42: 245-66
10. Lawton MT, Quiñones-Hinojosa A, Chang EF, Yu T. Thrombotic intracranial aneurysms: Classification scheme and management strategies in 68 patients. Neurosurgery. 2005. 56: 441-54
11. Lownie SP, Drake CG, Peerless SJ, Ferguson GG, Pelz DM. Clinical presentation and management of giant anterior communicating artery region aneurysms. J Neurosurg. 2000. 92: 267-77
12. Matsushima K, Kawashima M, Suzuyama K, Takase Y, Takao T, Matsushima T. Thrombosed giant aneurysm of the distal anterior cerebral artery treated with aneurysm resection and proximal pericallosal artery-callosomarginal artery end-to-end anastomosis: Case report and review of the literature. Surg Neurol Int. 2011. 2: 135-
13. Piepgras DG, Khurana VG, Whisnant JP. Ruptured giant intracranial aneurysms. Part II. A retrospective analysis of timing and outcome of surgical treatment. J Neurosurg. 1998. 88: 430-5
14. Sanai N, Zador Z, Lawton MT. Bypass surgery for complex brain aneurysms: An assessment of intracranial-intracranial bypass. Neurosurgery. 2009. 65: 670-83
15. Sugita M, Kinouchi H, Nishiyama Y, Kanemaru K, Yoshioka H, Horikoshi T. Direct clipping of a thrombosed giant cerebral aneurysm after thrombectomy without bleeding to minimize the temporary occlusion time-technical case report-. Neurol Med Chir (Tokyo). 2009. 49: 600-3
16. Spetzler RF, Kalani MYS, Nakaji P.editorsNeurovascular surgery. Thieme, New York: 2015. p.
17. Surdell DL, Hage ZA, Eddleman CS, Gupta DK, Bendok BR, Batjer HH. Revascularization for complex intracranial aneurysms. Neurosurg Focus. 2008. 24: E21-
18. Ture U, Hicdonmez T, Elmaci I, Peker S. Giant pericallosal artery aneurysm: Case report and review of the literature. Neurosurg Rev. 2001. 24: 151-5
19. Yasargil MG.editorsMicroneurosurgery. New York: Georg Thieme; 1984. I: