- Department of Neurosurgery, Johns Hopkins University School of Medicine, The Johns Hopkins Hospital, Baltimore, Maryland, USA
- Department of Neurosurgery, University of California, Irvine School of Medicine, UC Irvine Medical Center, California, USA
Correspondence Address:
Li-Mei Lin
Department of Neurosurgery, Johns Hopkins University School of Medicine, The Johns Hopkins Hospital, Baltimore, Maryland, USA
DOI:10.4103/sni.sni_13_17
Copyright: © 2017 Surgical Neurology International This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Bowen Jiang, Matthew T. Bender, Bima Hasjim, Frank P. K. Hsu, Rafael J. Tamargo, Judy Huang, Geoffrey P. Colby, Alexander L. Coon, Li-Mei Lin. Aneurysm treatment practice patterns for newly appointed dual-trained cerebrovascular/endovascular neurosurgeons: Comparison of open surgical to neuroendovascular procedures in the first 2 years of academic practice. 25-Jul-2017;8:154
How to cite this URL: Bowen Jiang, Matthew T. Bender, Bima Hasjim, Frank P. K. Hsu, Rafael J. Tamargo, Judy Huang, Geoffrey P. Colby, Alexander L. Coon, Li-Mei Lin. Aneurysm treatment practice patterns for newly appointed dual-trained cerebrovascular/endovascular neurosurgeons: Comparison of open surgical to neuroendovascular procedures in the first 2 years of academic practice. 25-Jul-2017;8:154. Available from: http://surgicalneurologyint.com/surgicalint-articles/aneurysm-treatment-practice-patterns-for-newly-appointed-dual%e2%80%91trained-cerebrovascularendovascular-neurosurgeons-comparison-of-open-surgical-to-neuroendovascular-procedures-in-the-first-2-yea/
Abstract
Background:The practice patterns of a hybrid open cerebrovascular/neuroendovascular (CVNV) neurosurgeon in early academic practice is unknown.
Methods:We performed a multi-institutional retrospective cohort study of patients with cerebral aneurysms that were treated within the first 24 months of the neurosurgeon’s practice.
Results:A total of 533 aneurysms were treated by the three senior authors within the first 24 months of their academic practice. Of these aneurysms, 172 were treated with microsurgical clipping, 191 with coiling, and 170 with flow diversion. Treatment in the setting of acute subarachnoid hemorrhage (SAH) occurred in 23% (122/533) of the aneurysms. Majority of the clipped aneurysms (70%, 121/172) were anterior cerebral artery (ACA), anterior communicating artery (ACOM), or middle cerebral artery (MCA) in location. In comparison, only 23% (82/361) of aneurysms treated with coiling or flow diversion therapy were ACA, ACOM, or MCA in location (P
Conclusion:Although the CVNV neurosurgeon treats cerebral aneurysms more commonly with neuroendovascular techniques, a third of the cerebral aneurysms are still selected for microsurgical clipping. Aneurysms located along the ACA/ACOM or MCA are the most frequent aneurysms reserved for microsurgical clipping. The CVNV neurosurgeon must be prepared to manage a high percentage of ACA/ACOM or MCA aneurysms microsurgically.
Keywords: Aneurysm, clipping, coiling, endovascular, hybrid, flow diversion
INTRODUCTION
Intracranial aneurysms are managed by microsurgical clipping and/or endovascular techniques, with the selection of treatment modality dependent on several patients and aneurysm characteristics. Key studies have established the relative safety and efficacy of the two techniques in terms of clinical and radiographic outcomes.[
Since the invention of the Guglielmi detachable coil in the 1990s, the field of neurointervention has blossomed, with now a vast array of coils, stents, balloons, flow diverters, and endovascular devices being utilized routinely in the treatment of intracranial aneurysms.[
To our knowledge, there are no clinical studies that quantitatively reviewed the practice patterns of a CVNV neurosurgeon during early independent practice. In this report, we present multi-institutional data from three dual-trained CVNV neurosurgeons during the first 2 years of practice postresidency. We review the case volume and aneurysm characteristics for both open and endovascular therapy.
MATERIALS AND METHODS
This study is an institutional-based, non-randomized, retrospective cohort study from prospectively collected databases. The study was conducted at two major academic institutions in the United States, one on the east coast and the other on the west coast. The cerebrovascular cases performed during the first 24 months of practice for the three senior authors, who are all CVNV neurosurgeons, were selected for review. All three CVNV neurosurgeons received 2 years of in-folded, dedicated endovascular neurosurgery fellowship training at the Johns Hopkins Hospital.
Cerebrovascular cases that were specific to aneurysm treatment were identified for analysis. Data were collected with respect to treatment modality (flow diversion versus coiling with and without use of adjunctive versus craniotomy), aneurysm location, and rupture status at presentation. Cases performed for treatment of other cerebrovascular pathologies (e.g., dural arteriovenous fistulas, arteriovenous malformations, etc.) and diagnostic cerebral angiography were not included in the analysis. The same aneurysm treated with multistage stent-assisted coiling was counted as one case. Data were presented as counts. Statistical analysis was performed on proportions of counts and a Fisher exact test was used to determine statistical significance between open microsurgical clipping and endovascular therapy (inclusive of both flow diversion and coil/stenting). A probability value of <0.05 was considered to be statistically significant.
RESULTS
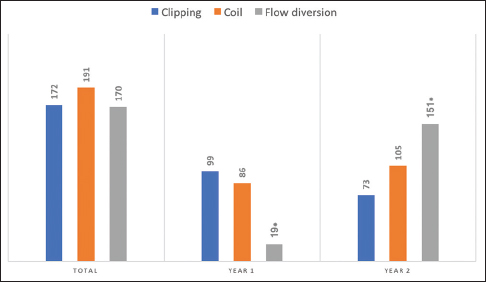
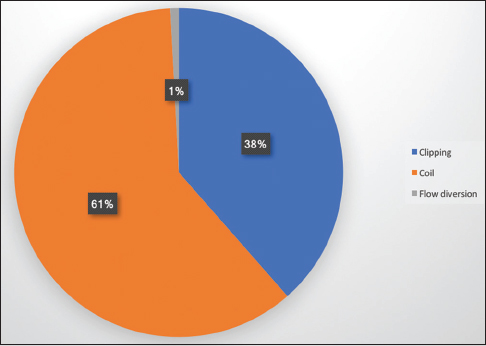
A total of 533 aneurysms were treated by the three senior authors [(Geoffrey Colby (GPC), Alexander Coon (ALC), Li-Mei Lin (LML)] during their first 2 years of practice at their respective academic institutions (GPC July 2013 to June 2015, ALC July 2010 to June 2012, LML August 2014 to July 2016). Microsurgical clipping was performed for the treatment of 172 (32%) aneurysms while a neuroendovascular approach was undertaken for 361 (68%) aneurysms [
Of the 533 aneurysm cases treated, 122 (23%) presented in the ruptured setting with acute subarachnoid hemorrhage (SAH). For these SAH cases, 75/122 (61%) were treated endovascularly with coiling in all, but one case where the aneurysm was treated with a flow diverter in a delayed fashion within 1 month of the SAH [
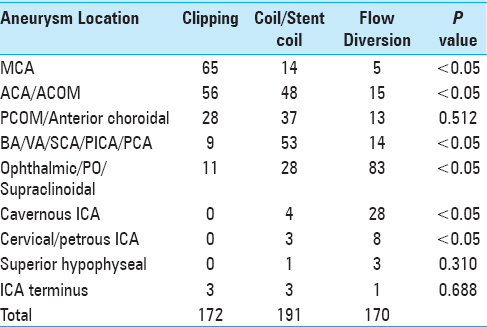
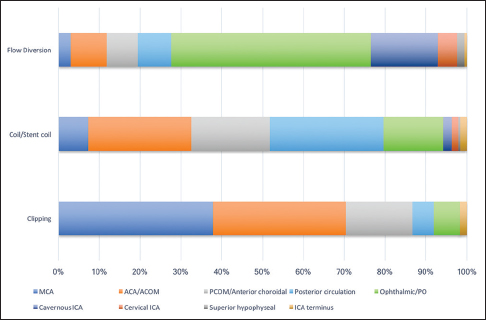
When all microsurgically treated aneurysm cases (both ruptured and unruptured) were considered, 70% (121/172) were located along the ACA, ACOM, or MCA [
DISCUSSION
Recent advancements in neuroendovascular techniques, including the introduction of flow diversion, have created increasing demand for the dual trained CVNV neurosurgeon. Initially, this training approach was met with skepticism, as many questioned whether a dual trained neurosurgeon would have the technical capacity and experience to safely perform both open and endovascular techniques. This skepticism is garnered from literature that suggest higher case volume (>20 cases/year) is associated with improved clinical outcomes, particularly in the setting of aneurysmal SAH.[
As endovascular techniques continue to evolve, fewer aneurysms are selected for microsurgical clipping.[
The findings from this study also suggest a trend towards more endovascular management of aneurysms in the second year of practice for a newly minted CVNV neurosurgeon, despite known potential influencers on junior attending practice preferences from institutional bias and senior faculty experience. In our study, all three CVNV neurosurgeons underwent endovascular training at the same institution, one that historically preferred open microsurgical clipping. The senior partners for all three practitioners only practiced open microsurgery and do not have endovascular training. Despite the dominant, open vascular influences, the trend towards endovascular management persisted, likely as a true reflection of current clinical trends. Particularly, this trend was statistically significant for the increased utilization flow diversion techniques. This trend could be confounded by the fact that flow diversion was still in its infancy during one of the senior author’s first year in practice, during which time the PED was not widely available at the institution. The lower volume of flow diversion during year 1 of practice could also be attributed to proctoring requirements prior to off-label treatments.
In the current study, the majority of the cases treated by the senior authors were elective, unruptured aneurysms. As such, only about 20% of the patients treated were in the setting of ruptured aneurysmal SAH. Most junior CVNV neurosurgeons would be expected to have higher numbers of non-elective cases early in their practice. The current data reflect that of academic centers with high referral patterns, thus may skew the practice pattern towards higher elective volume.
CONCLUSION
Although there is an increasing trend towards endovascular treatment of majority of cerebral aneurysms by the hybrid cerebrovascular neurosurgeon, this study demonstrates that a significant number of MCA and ACA aneurysms are still selected for microsurgical clipping by the CVNV neurosurgeon. The hybrid CVNV neurosurgeon must be prepared to manage a high percentage of ACA/MCA aneurysms with open microsurgical techniques in both unruptured and ruptured settings.
Financial support and sponsorship
Nil.
Conflicts of interest
ALC is a proctor for the Woven Endobridge (WEB) device (Sequent Medical, Aliso Viejo, CA), a proctor for the Surpass device (Stryker Neurovascular, Fremont, CA) and a consultant for Stryker Neurovascular, a proctor for the Pipeline Embolization Device (Medtronic Neurovascular, Irvine, CA) and a consultant for Medtronic, and a proctor for the FRED device (MicroVention, Tustin, CA) and consultant for MicroVention. GPC is a consultant for Medtronic, MicroVention and participates in clinical trials for Medtronic and Stryker. LML is a proctor for the Pipeline Embolization Device (Medtronic Neurovascular, Irvine, CA), a consultant for MicroVention and participates in clinical trial for Stryker. The other authors have no conflict of interest. No author received financial support in conjunction with the generation of this submission.
References
1. Alshafai N, Falenchuk O, Cusimano MD. International differences in the management of intracranial aneurysms: Implications for the education of the next generation of neurosurgeons. Acta Neurochir (Wien). 2015. 157: 1467-75
2. Bekelis K, Gottlieb D, Bovis G, Su Y, Tjoumakaris S, Jabbour P. Unruptured cerebral aneurysm clipping: Association of combined open and endovascular expertise with outcomes. J Neurointerv Surg. 2016. 8: 977-81
3. Bekelis K, Gottlieb D, Labropoulos N, Su Y, Tjoumakaris S, Jabbour P. The impact of hybrid neurosurgeons on the outcomes of endovascular coiling for unruptured cerebral aneurysms. J Neurosurg. 2017. 126: 29-35
4. Darsaut TE, Jack AS, Kerr RS, Raymond J. International Subarachnoid Aneurysm Trial-ISAT part II: Study protocol for a randomized controlled trial. Trials. 2013. 14: 156-
5. de Vries J, Boogaarts HD. Treatment of patients with ruptured aneurysm by neurosurgeons that perform both open surgical and endovascular techniques is safe and effective: Results of a single centre in Europe. Acta Neurochir (Wien). 2014. 156: 1259-66
6. Harbaugh RE, Agarwal A. Training residents in endovascular neurosurgery. Neurosurgery. 2006. 59: S277-81
7. Henkes H, Fischer S, Weber W, Miloslavski E, Felber S, Brew S. Endovascular coil occlusion of 1811 intracranial aneurysms: Early angiographic and clinical results. Neurosurgery. 2004. 54: 268-80
8. Jiang B PM, Colby GP, Coon AL, Lin LM. Cerebral aneurysm treatment: Modern neurovascular techniques. Stroke Vasc Neurol. 2016. 1: 93-100
9. Kerr R, Molyneux A. The barrow ruptured aneurysm trial and international subarachnoid aneurysm trial. J Neurosurg. 2013. 118: 478-80
10. Lanzino G, Rabinstein AA. Endovascular neurosurgery in the United States: A survey of 59 vascular neurosurgeons with endovascular training. World Neurosurg. 2011. 75: 580-5
11. Molyneux A, Kerr R, Stratton I, Sandercock P, Clarke M, Shrimpton J. International Subarachnoid Aneurysm Trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: A randomised trial. Lancet. 2002. 360: 1267-74
12. Park HK, Horowitz M, Jungreis C, Genevro J, Koebbe C, Levy E. Periprocedural morbidity and mortality associated with endovascular treatment of intracranial aneurysms. AJNR Am J Neuroradiol. 2005. 26: 506-14
13. Raymond J, Kotowski M, Darsaut TE, Molyneux AJ, Kerr RS. Ruptured aneurysms and the International Subarachnoid Aneurysm Trial (ISAT): What is known and what remains to be questioned. Neurochirurgie. 2012. 58: 103-14
14. Richling B, Lasjaunias P, Byrne J, Lindsay KW, Matge G, Trojanowski T. Standards of training in endovascular neurointerventional therapy: As approved by the ESNR, EBNR, UEMS Section of Neurosurgery and EANS (February 2007). Enclosed the standards of practice as endorsed by the WFITN. Acta Neurochir (Wien). 2007. 149: 613-6
15. Rush B, Romano K, Ashkanani M, McDermid RC, Celi LA. Impact of hospital case-volume on subarachnoid hemorrhage outcomes: A nationwide analysis adjusting for hemorrhage severity. J Crit Care. 2017. 37: 240-3
16. Sanai N, Caldwell N, Englot DJ, Lawton MT. Advanced technical skills are required for microsurgical clipping of posterior communicating artery aneurysms in the endovascular era. Neurosurgery. 2012. 71: 285-94
17. Sauvageau E, Hopkins LN. Training in cerebrovascular disease: Do we need to change the way we train residents?. Neurosurgery. 2006. 59: S282-6
18. Wong JM, Ziewacz JE, Ho AL, Panchmatia JR, Kim AH, Bader AM. Patterns in neurosurgical adverse events: Open cerebrovascular neurosurgery. Neurosurg Focus. 2012. 33: E15-