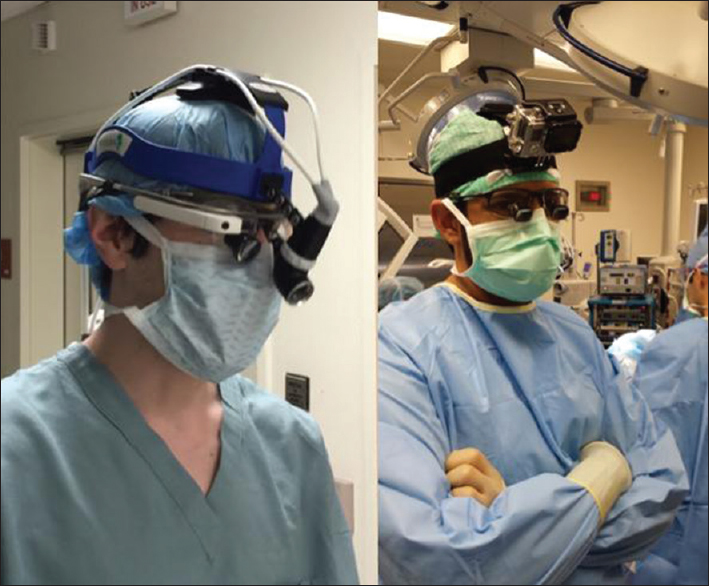
- School of Medicine, Department of Neurological Surgery, University of California, Irvine, California, USA
- Division of Neurotrauma, Department of Neurological Surgery, University of California, Irvine, California, USA
Correspondence Address:
Jefferson W. Chen
School of Medicine, Department of Neurological Surgery, University of California, Irvine, California, USA
Division of Neurotrauma, Department of Neurological Surgery, University of California, Irvine, California, USA
DOI:10.4103/sni.sni_277_16
Copyright: © 2017 Surgical Neurology International This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Ronald Sahyouni, Omid Moshtaghi, Diem Kieu Tran, Sean Kaloostian, Ramin Rajaii, David Bustillo, Jefferson W. Chen. Assessment of Google Glass as an adjunct in neurological surgery. 26-Apr-2017;8:68
How to cite this URL: Ronald Sahyouni, Omid Moshtaghi, Diem Kieu Tran, Sean Kaloostian, Ramin Rajaii, David Bustillo, Jefferson W. Chen. Assessment of Google Glass as an adjunct in neurological surgery. 26-Apr-2017;8:68. Available from: http://surgicalneurologyint.com/surgicalint-articles/assessment-of-google-glass-as-an-adjunct-in-neurological-surgery/
Abstract
Background:We assess Google Glass (“Glass”) in improving postoperative review (“debriefing”) and augmenting education in Neurological Surgery at a tertiary academic medical center.
Methods:This was a prospective study. Participants were patients of Neurological Surgery physicians at a Tertiary Care Level 1 Academic Trauma Center. Resident physicians received a pre-questionnaire immediately following surgery. Next, the resident and attending physicians debriefed by reviewing the Glass operative recording. Then, residents completed a 4-part post-questionnaire. Questions 1–3 assessed: (1) the residents’ comfort level with the procedure, (2) the quality of education provided by their superiors, and (3) their comfort level in repeating the operation. Question 4 assessed: (4) the perceived benefit of debriefing using Glass.
Results:Twelve surveys were collected. Scores were based on a 5-point Likert scale, with a higher score corresponding to a more positive response. For Questions 1–3, the average pre- and post-questionnaire scores were 3.75 and 4.42, respectively (P <.05 for question the average post-questionnaire score was suggesting that postoperative glass review improved their technical understanding of procedure.>
Conclusions:Glass significantly improved neurosurgery residents’ comfort level and quality of training, and provided a high fidelity, reliable, and modifiable tool that enhanced residents’ understanding, expertise, and educational experience. Of note, certain limitations such as variable battery life, variable image quality, and subpar compatibility with surgeon loupes must still be overcome for Glass to become a realistic addition to neurosurgical education.
Keywords: Education, Glass, interactive, neurosurgery, resident, video
INTRODUCTION
Cameras were first employed in medicine in 1840 when Alfred Donne obtained pictures of bones, red blood cells, and teeth using a primitive microscope.[
With significant advancements in technology, reductions in size have enabled the feasibility of using wearable technology in the operating room (OR).[
In the past, Glass has been incorporated in surgical and nonsurgical fields including plastic surgery, cardiology, pediatric surgery, and otolaryngology.[
First, we aimed to identify neurosurgeons’ levels of comfort using Glass. The technological specifications and method of use bear a moderate learning curve. In addition, some authors have described its physical incompatibility with the surgical loupes required for operation [
Second, we aimed to assess the utility of Glass for postsurgical review of point-of-view (POV) recordings. At present, the microscale anatomy and time urgency of neurosurgical operations often makes it difficult to optimally educate residents. Glass may improve the attending physicians’ ability to more effectively train residents by recording the procedures for subsequent review and discussion.
Third, we sought to identify the overall impact of Glass on residents’ medical training and technical understanding of procedures. In addition to postoperative surgical review, a resident wearing Glass can live stream the surgical field from their POV to their superiors, who may fix errors and provide real-time modifications, live or remotely. Further, we used Glass as a cost-effective method to create educational vignettes, which are then posted remotely online for educational purposes.
Finally, we wished to demonstrate the benefit of Glass in optimizing interdisciplinary dynamics and communication in the OR. Glass-mediated communication eliminates background noise, provides unique vantage points, and on-screen remote guidance by team members not directly involved in the surgery.
MATERIALS AND METHODS
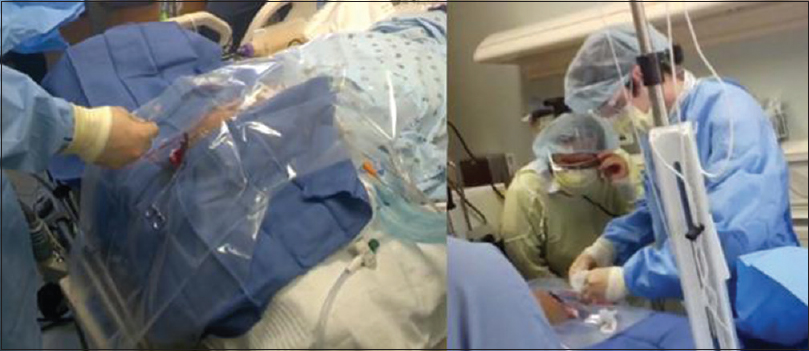
This prospective cross-sectional study was performed with IRB approval. Prior to the first operation, resident physicians received a tutorial on how to use Glass. Residents were asked to wear Glass during the surgery. Resident physicians received a pre-questionnaire immediately following the surgery. Next, resident and attending physicians debriefed by reviewing the Glass operative recording. Then, residents completed a post-questionnaire consisting of identical questions. Questions 1–3 assessed: (1) The residents’ comfort level with the procedure; (2) the quality of education provided by their superiors; and (3) their comfort level in repeating the operation. Question 4 assessed: (4) The perceived benefit of debriefing using Glass.
Technological specifications of Glass
Glass connects to a phone via Bluetooth or wireless network, enabling hands-free Internet access. Using a 720 p high definition camera and microphone, Glass records video and audio with voice commands.[
RESULTS
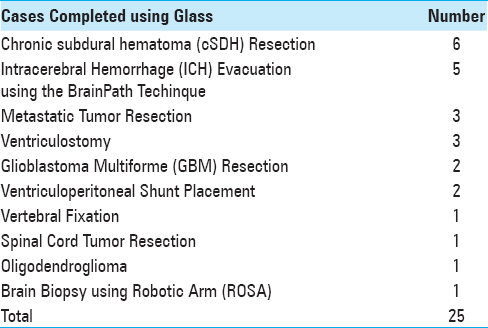
Twenty-five procedures were completed on twenty-five unique patients [
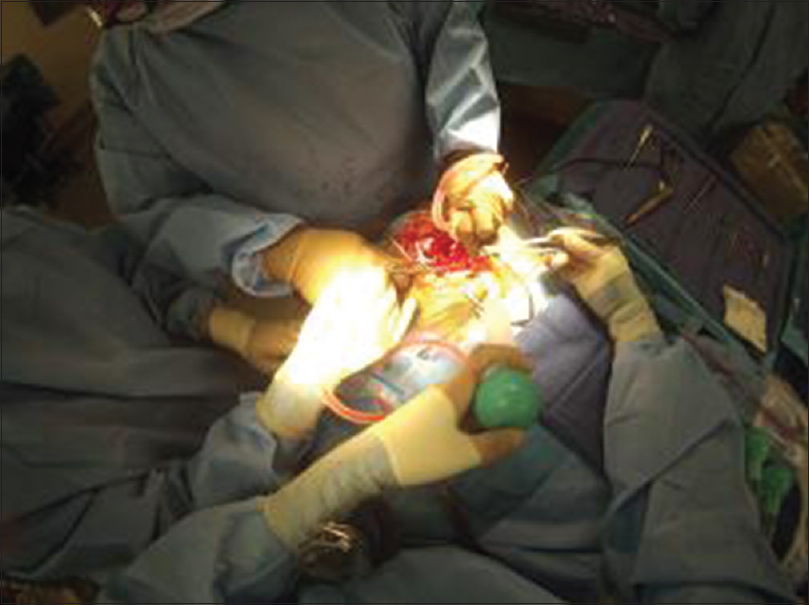
In our study, GoPro technology (“GoPro”) was implemented to better illustrate Glass's technical deficiencies and overall feasibility in the OR. The GoPro is a high-definition, waterproof, and shockproof video recording device. It has gained popularity in extreme sports as well as clinical procedures due to its compact size, rugged durability, and impressive video quality. Here, GoPro was shown to have superior image quality, greater range of motion, improved battery life, and more straightforward physical compatibility with surgical loupes [
DISCUSSION
Benefits – education, interdisciplinary communication, and surgical review
Glass has a variety of beneficial uses within the realms of medical education, interdisciplinary hospital communication, and local as well as remote surgical review. In regard to medical education, the training of residents is often difficult due to the complexities, microsurgical anatomy and time-pressures of the operating room. This is particularly the case within the field of neurosurgery. As a result, techniques in simulation and virtual reality have substantially improved the quality of this education.[
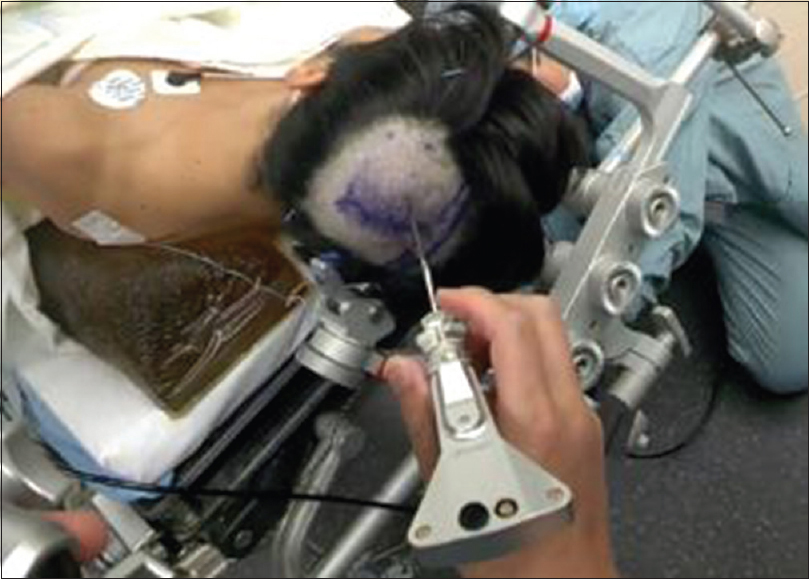
Glass was found to improve multidisciplinary communication and team dynamics in numerous ways. First, by using the live-streaming capabilities, it is possible to project the surgeon's view onto a laptop or the operating room monitors to allow other members of the team (circulating nurses, anesthesiologists) to follow the course of the surgery. The attending neurosurgeon was also able to view and critique the procedure at a distance without the impression of intruding on the residents’ space. Second, Glass aided in staff communication within the OR. During a brain biopsy procedure, using the ROSA (Montpellier, France) robotic arm,[
Finally, in addition to enabling opportunities for resident education within the hospital, Glass can also provide a platform for remote learning. Glass can allow surgeons worldwide to create a database of educational surgical vignettes. These vignettes can be posted for public educational purposes. Viewers may watch first-hand surgical POV recordings to gain further experience observing surgeries and refining techniques. These recordings are particularly easy to edit, allowing the condensation of a long, often tedious, surgery into a one or two minute video ideal for remote learning and education.
Limitations of Glass – battery life, physical convenience, sterility, and image quality
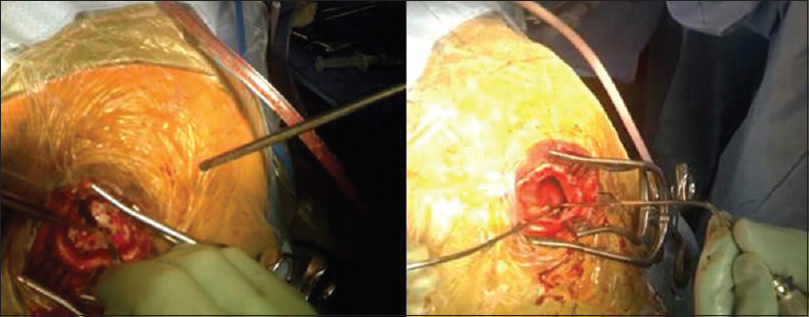
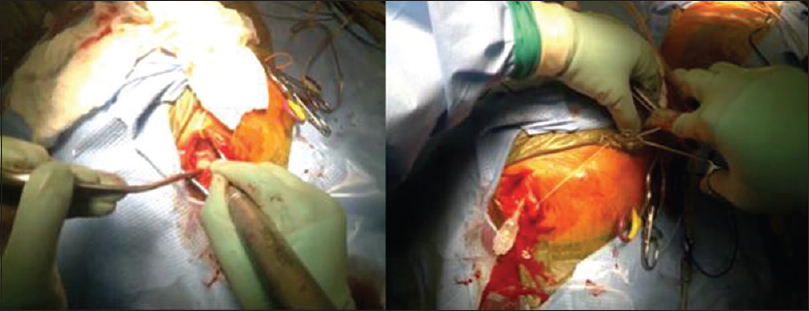
While it has many functional features and capabilities, Glass has limitations that become particularly relevant in neurosurgery. First, due to the small structures of cranial cavity, and lack of color contrast, it is difficult to visualize the full depth of anatomy on film. Second, the bright lights of the OR in conjunction with Glass's camera sometimes cause over-exposure of the images making the videos difficult to view. For instance, recording of a cSDH evacuation did show the structures of the galea, cranium, and dura, but could not adequately distinguish subdural membrane from the underlying dura and arachnoid layers [
Comparison with GoPro
Paro et al. also compared Glass with GoPro. They found GoPro to be superior in recording surgical footage. Due to GoPro's ability to pivot on its horizontal axis, users did not have to engage in cervical flexion to focus the picture on the middle of the screen. Graphics resolution was also superior to Glass, with the GoPro device capable of capturing anatomy in greater detail [
The Glass recording can be activated by voice commands alone, limiting the user's need for non-sterile physical support. Finally, battery life was insufficient in both devices; Paro et al. found the GoPro to last 2 hours while the Glass only lasted 1 hour for continuous high-definition recording. Our experiences confirmed this finding. In summary, the GoPro provided superior image quality, lasted longer, could be worn with the loupes while operating, but required a non-sterile team member to activate and end all pictures or video.
CONCLUSION
Our study found Google Glass to have numerous benefits as an educational tool. We believe it can serve as a useful method to capture POV surgical footage for debriefing and creation of educational vignettes. Our survey, though subjective in nature, demonstrated significant improvement in resident medical education in Neurological Surgery at UCIMC, and residents felt more comfortable with their surgical skills following debriefing. Furthermore, despite its moderate learning curve, physicians felt comfortable using the device. In our experience, Glass also improved interdisciplinary communication within the OR, across the hospital, and with physicians in remote locations. Glass can also provide the opportunity to create a database of publically available educational surgical vignettes. Overall, we optimistically recommend further evaluation of Google Glass as an educational tool in neurosurgery; however, this statement rests upon the assumption that technical limitations are first addressed. These limitations include physical compatibility with loupes and headlights common in certain subspecialties, limited battery life, issues with sterility, and inferior image quality when compared to similar mobile technology.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Albrecht UV, Von Jan U, Kuebler J, Zoeller C, Lacher M, Muensterer OJ. Google Glass for documentation of medical findings: Evaluation in forensic medicine. J Med Internet Res. 2014. 16: e53-
2. Aljamal YN, Ali SM, Ruparel RK, Brahmbhatt RD, Yadav S, Farley DR. The rationale for combining an online audiovisual curriculum with simulation to better educate general surgery trainees. Surgery. 2014. 156: 723-8
3. Armstrong DG, Rankin TM, Giovinco NA, Mills JL, Matsuoka Y. A heads-up display for diabetic limb salvage surgery: A view through the google looking glass. J Diabetes Sci Technol. 2014. 8: 951-6
4. Benninger B. Google Glass, ultrasound and palpation: The anatomy teacher of the future?. Clin Anat. 2015. 28: 152-5
5. Chai PR, Wu RY, Ranney ML, Bird J, Chai S, Zink B. Feasibility and Acceptability of Google Glass for Emergency Department Dermatology Consultations. JAMA Dermatol. 2015. 151: 794-6
6. Chen JW, Paff MR, Abrams-Alexandru D, Kaloostian SW. Decreasing the Cerebral Edema Associated with Traumatic Intracerebral Hemorrhages: Use of a Minimally Invasive Technique. Acta Neurochir Suppl. 2016. 121: 279-84
7. Davis CR, Rosenfield LK. Looking at plastic surgery through Google Glass: Part 1. Systematic review of Google Glass evidence and the first plastic surgical procedures. Plast Reconstr Surg. 2015. 135: 918-28
8. Ding D, Przybylowski CJ, Starke RM, Sterling Street R, Tyree AE, Webster Crowley R. A minimally invasive anterior skull base approach for evacuation of a basal ganglia hemorrhage. J Clin Neurosci. 2015. 22: 1816-9
9. Feng S, Caire R, Cortazar B, Turan M, Wong A, Ozcan A. Immunochromatographic diagnostic test analysis using Google Glass. ACS Nano. 2014. 8: 3069-79
10. Gonzalez-Martinez J, Vadera S, Mullin J, Enatsu R, Alexopoulos AV, Patwardhan R. Robot-assisted stereotactic laser ablation in medically intractable epilepsy: Pperative technique. Neurosurgery. 2014. 10: 167-72
11. Jeroudi OM, Christakopoulos G, Christopoulos G, Kotsia A, Kypreos MA, Rangan BV. Accuracy of remote electrocardiogram interpretation with the use of Google Glass technology. Am J Cardiol. 2015. 115: 374-7
12. Kantor J. First look: Google Glass in dermatology, Mohs surgery, and surgical reconstruction. JAMA Dermatol. 2014. 150: 1191-
13. Linder TE, Simmen D, Stool SE. Revolutionary inventions in the 20th century. The history of endoscopy. Arch Otolaryngol Head Neck Surg. 1997. 123: 1161-3
14. Moshtaghi O, Kelley KS, Armstrong WB, Ghavami Y, Gu J, Djalilian HR. Using Google Glass to solve communication and surgical education challenges in the operating room. Laryngoscope. 2015. 125: 2295-7
15. Muensterer OJ, Lacher M, Zoeller C, Bronstein M, Kübler J. Google Glass in pediatric surgery: An exploratory study. Int J Surg. 2014. 12: 281-9
16. Paro JA, Nazareli R, Gurjala A, Berger A, Lee GK. Video-based self-review: Comparing Google Glass and GoPro technologies. Ann Plast Surg. 2015. 74: S71-4
17. Parslow GR. Commentary: Google glass: A head-up display to facilitate teaching and learning. Biochem Mol Biol Educ. 2014. 42: 91-2
18. Piromchai P, Avery A, Laopaiboon M, Kennedy G, O’leary S. Virtual reality training for improving the skills needed for performing surgery of the ear, nose or throat. Cochrane Database Syst Rev. 2015. 9: CD010198-
19. Sibley P, Martineau D. Through the Looking Glass: Google Glass and the Future of Hand Surgery. AAOS Now. 2014. 8: 16-
20. Tobin W. Alfred Donné and Léon Foucault: The first applications of electricity and photography to medical illustration. J Vis Commun Med. 2006. 29: 6-13
21. Vadera S, Chan A, Lo T, Gill A, Morenkova A, Phielipp NM. Frameless Stereotactic Robot-Assisted Subthalamic Nucleus Deep Brain Stimulation: Case Report. World Neurosurg. 2017. 97: e11-762.e14