- Department of Neurosurgery, University of South Florida, Tampa, Florida, USA
- NuVasive, Inc, San Diego, California, USA
- Department of Neurosurgery, University of Virginia, Charlottesville, Virginia, USA
Correspondence Address:
Jacob Januszewski
Department of Neurosurgery, University of South Florida, Tampa, Florida, USA
DOI:10.4103/sni.sni_44_17
Copyright: © 2017 Surgical Neurology International This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Jacob Januszewski, Joshua M. Beckman, Jeffrey E. Harris, Alexander W. Turner, Chun Po Yen, Juan S. Uribe. Biomechanical study of rod stress after pedicle subtraction osteotomy versus anterior column reconstruction: A finite element study. 06-Sep-2017;8:207
How to cite this URL: Jacob Januszewski, Joshua M. Beckman, Jeffrey E. Harris, Alexander W. Turner, Chun Po Yen, Juan S. Uribe. Biomechanical study of rod stress after pedicle subtraction osteotomy versus anterior column reconstruction: A finite element study. 06-Sep-2017;8:207. Available from: http://surgicalneurologyint.com/surgicalint-articles/biomechanical-study-of-rod-stress-after-pedicle-subtraction-osteotomy-versus-anterior-column-reconstruction-a-finite-element-study/
Abstract
Background:In an effort to minimize rod fractures and nonunion in pedicle subtraction osteotomy (PSO) constructs, surgeons have adopted multirod constructs and interbody cages. Anterior column realignment (ACR) with posterior column osteotomies is a minimally invasive alternative to PSO in sagittal balance correction, however, there is a paucity of evidence with respect to rod survival.
Methods:Three-dimensional (3D) finite-element-model of a T12-sacrum spine segment was used to compare a 25° PSO at L3 and an ACR with a posterior column osteotomy and 30° hyperlordotic interbody cage at L3–4. The amount of overall T12–S1 lordosis correction was the same for each condition. Each simulation included cobalt chromium alloy primary rods with: (1) PSO; (2) PSO with an interbody cage (IB) at L2–3 (PSO+IB); (3) PSO with accessory (A) rods and IB at L2–3 (PSO+IB+A); (4) PSO with satellite (S) rods and IB at L2–3 (PSO+IB+2S); (5) ACR; 6) ACR with satellite rods (ACR + 2S). A 400 N follower preload was simulated for each condition.
Results:PSO condition had the largest rod stress of 286 MPa in flexion. Adding interbody support reduced the rod stress by 15%. The 4-rod constructs further reduced rod stress, with the satellite rod condition facilitating the largest reduction. The rod stress in the ACR+2S was equivalent to the PSO+2S, with 50% reduction in rod stress.
Conclusion:The rod stress with an ACR was comparable to a PSO coupled with interbody support. These results suggest an ACR is a viable MIS alternative to a PSO without the need for a large posterior osteotomy.
Keywords: Anterior column realignment, finite element analysis, MIS, pedicle subtraction osteotomy, rod fracture
INTRODUCTION
Pedicle subtraction osteotomies (PSOs) can enable significant corrections of alignment in adult deformity surgery, yielding 25–40° of lordosis at a single level depending on the technique.[
MATERIALS AND METHODS
A nonlinear three-dimensional (3D) finite element model of a T12-sacrum spine segment was created using geometric data derived from computed tomography (CT) scan of a cadaveric spine.[
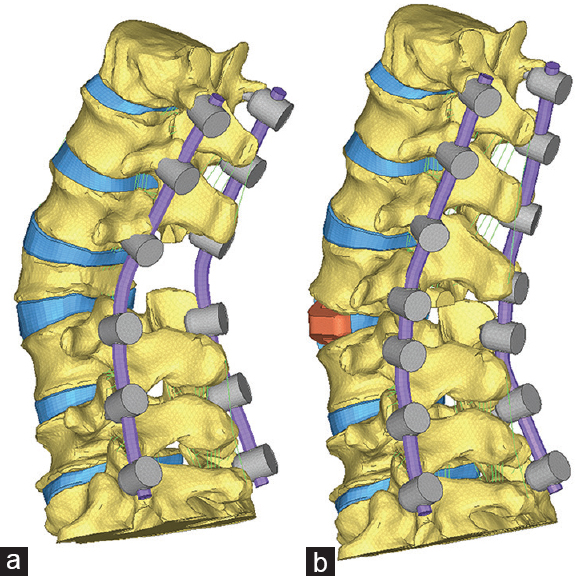
The geometry was modified to approximate a 25° PSO at L3 or an ACR with a 30° interbody cage and Smith-Petersen osteotomy (SPO) at L3–L4. Each condition had the same degree of lordosis restoration over the T12–S1 segment. The osteotomies were created by removing all the posterior elements of L3 using a 25° wedge and then closing the osteotomy. Cobalt chromium alloy bilateral rods with segmental fixation, classified as primary rods, ranged T12–S1. The presence of supplemental rods across the index level was classified as follows: accessory rods, which were connected to the primary rods via cross-connectors, or satellite rods, which were independently anchored to the adjacent vertebrae.
Model validation
The stiffness of each spinal motion segment in the primary loading directions (flexion-extension, lateral bending, and axial rotation) is primarily controlled by the material properties of the intervertebral disc and spinal ligaments. The intact, uninstrumented spine model was validated by comparing the segmental stiffness of the model with experimental in-vitro results from literature.[
Test conditions
A 400 N follower preload combined with unconstrained pure 7.5 N·m moments in flexion, extension, lateral bending, and axial rotation was applied to the superior endplate of T12. The sacrum was restricted from all motions via rigid anchors. A pure moment was used to load the model as it has two primary advantages: it is independent of spinal geometry as the applied moment on the proximal vertebrae is applied equally to all segments in the spine and the pure moment remains unchanged as the spine deforms during testing.[
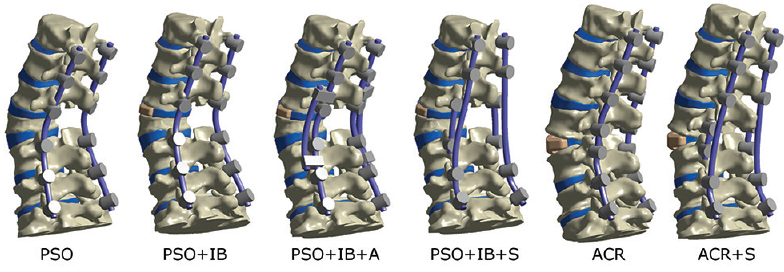
PSO construct with bilateral primary rods from T12–S1 (PSO) PSO with an interbody cage (IB) at L2–L3 and bilateral primary rods from T12–S1 (PSO+IB) PSO with an interbody cage at L2–L3, primary rods from T12–S1, and bilateral accessory rods (A) spanning L1–L2 to L4–L5 (PSO+IB+A) PSO with an interbody cage at L2–L3, primary rods from T12–S, and bilateral satellite rods (S) from L2–L4 (PSO+IB+2S) ACR construct with a 30° interbody cage L3–L4 and bilateral primary rods from T12–S1 (ACR) ACR construct with a 30° interbody cage L3–L4, bilateral primary rods from T12–S1, and bilateral satellite rods from L3–L4 (ACR+S).
The von Mises stress distribution in the primary and supplemental rods under maximum load (7.5 N·m) was calculated for each test construct in each loading direction. Maximum rod stress was determined.
RESULTS
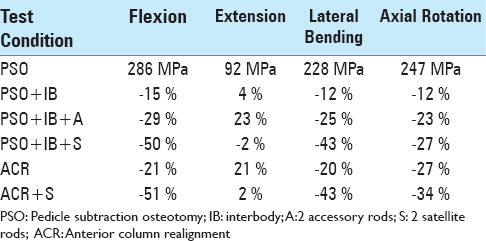
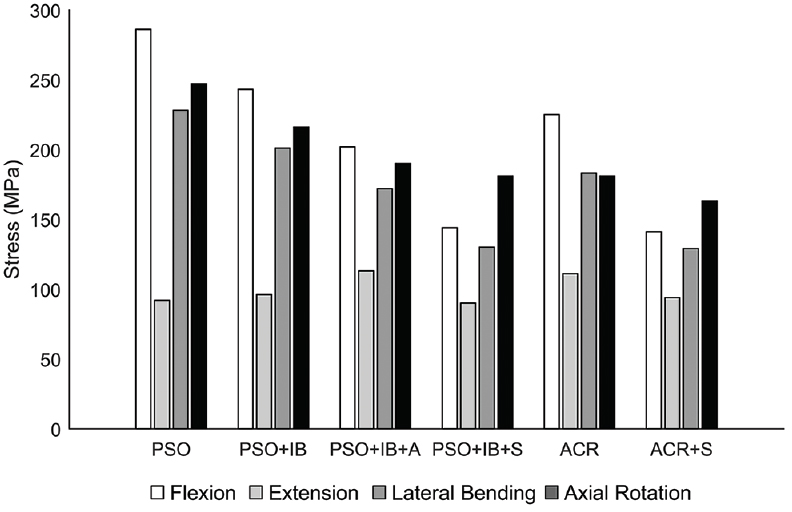
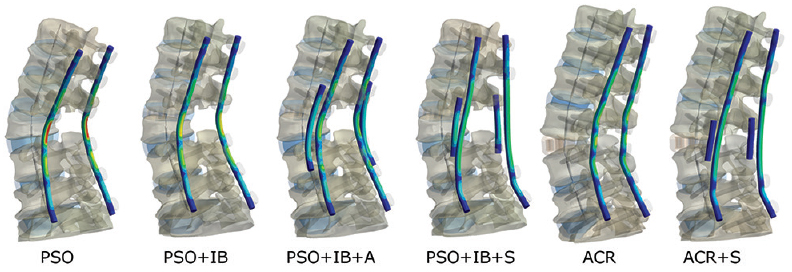
The largest rod stress of 286 MPa was observed with the PSO construct under the flexion loading condition [
Figure 4
Maximum von Mises stress contour plots for each rod. The stress scaling is the same for all images (0-300 MPa). Geometric scaling is also identical, however ACR is a lengthening procedure. PSO denotes pedicle subtraction osteotomy; IB, interbody; A, 2 accessory rods; S, 2 satellite rods; ACR, anterior column realignment
In extension, the rod stress for a PSO was 92 MPa. The PSO+IB and PSO+IB+A conditions, respectively, resulted in 4% and 23% increases in stress. The ACR condition resulted in a 21% increase in stress compared to PSO. The satellite rod condition resulted in the smallest changes in stress, with the PSO+IB+S having a 2% decrease, while the ACR+2S had a 2% increase in stress relative to a PSO under 7.5 N·m of extension.
In lateral bending, the rod stress for a PSO was 228 MPa. The PSO+IB and PSO+IB+A conditions, respectively, resulted in 12% and 25% decreases in stress. The ACR condition resulted in a 20% decrease in stress. The satellite rod condition resulted in equivalent changes in stress, with the PSO+IB+S and ACR+2S each having a 43% decrease in stress.
In axial rotation, the rod stress for a PSO was 247 MPa. The PSO+IB and PSO+IB+A conditions, respectively, resulted in 12% and 23% decreases in stress. The ACR condition resulted in a 27% decrease in stress. The satellite rod condition resulted in decreased stress by 27% for the PSO+IB+S condition and by 34% for the ACR+2S condition.
DISCUSSION
Prior studies and cadaveric investigations demonstrated that sagittal correction with an ACR and posterior column osteotomies was comparable to that of a PSO.[
The highest rod fracture rates reported in literature are with the classic PSO techniques, and the lowest reported are those with satellite rods and interbody supplementation.[
In our FEA study, we compared four PSO constructs and analyzed the mechanical stress on the rods for each one. Alternative physical methods to study rod loading, such as strain gages, only provide strain information at discrete gage locations. A finite element model is advantageous as it provides the stress distribution along the entire rod and eliminates specimen variability. A PSO model representing 25° of lordosis at L3 was used for all constructs. A PSO without an interbody showed the highest stress on the rods, and this stress was minimized somewhat by adding an interbody at the superior disc space. A PSO with interbody and accessory rods further minimized rod stress, however, the biggest reduction in stress was achieved in a PSO model with an interbody and two satellite rods. Use of multirod system versus a 2-rod system has been previously described in successfully minimizing instrumentation failure at the osteotomy site as well as the rates of pseudoarthrosis,[
The mechanical stress reduction of an ACR with satellite rods was equivalent to a PSO interbody construct with satellite rods (PSO+IB+S), both reducing rod stress by 50% in flexion. The mechanical stress of an ACR without satellite rods was 21% less than that of the classic PSO but still 8% higher than that of a PSO interbody construct with accessory rods (PSO+IB+A) in flexion loading. Interestingly, when comparing a PSO with interbody support (PSO+IB) and primary rods with an ACR without satellite rods, the difference was only 6% (15% vs. 21% stress reduction, respectively). The results of our FEA show that the ACR may have a biomechanical support advantage over the un-supplemented PSO and that both supported with satellite rods may be comparable in rod stress reduction. It is yet to be determined if these constructs have equivalent clinical benefit in terms of hardware failure and pseudoarthrosis rates.
Complex spinal deformities can accurately be reconstructed and studied using FEA, with loading conditions similar to that of cadaveric studies. Such models are a good option for predicting possible clinical outcomes in hard to design or lengthy case-control studies or randomized trials. Results from FEA can be used to design even better clinical studies to follow as they cannot completely account for all components effecting mechanical behavior of the spinal column. Gravity, postural control by the muscular system, and the effect of demographics, age, and race were not considered and may affect the analysis. Conclusions from finite element studies cannot be directly correlated clinically, but can be a good predictor of trends that may correlate with clinical outcomes and provide an impetus for further investigation.
There are several important limitations of our study. We did not address a difference in rigidity of different kinds of rods. Stress reductions may differ depending on whether cobalt-chromium, titanium, or steel alloy rods are used. In addition, rod fracture occurs after cyclic loading which creates an alternating stress on the rod. Because we did not perform cyclic loading, it is not possible to tell how soon the rod would fracture or predict the clinical risk of instrumentation failure. It is possible that our results would be magnified or changed over time with cyclic loading analysis. While the timing issue through cyclic loading was not addressed, the invaluable information of stress reduction with supplemental rods or alternative techniques such as MIS ACR cannot be overlooked. The challenge of creating rod fractures in a cadaveric model has been demonstrated previously,[
CONCLUSION
FEA showed significant (50% in flexion) primary rod stress reduction in a PSO with interbody and 4-rod satellite construct compared to a PSO without interbody and 2-rod construct, which may reduce risk of rod fracture in vivo with the 4-rod construct. Interestingly, ACR with a 4-rod satellite construct had comparable stress reduction to the PSO 4-rod satellite construct in all loading directions. ACR with a 4-rod construct is an excellent MIS alternative to a more morbid surgery requiring large osteotomies. This FEA needs to be further confirmed in a clinical scenario in a prospective study and long-term follow up, which is currently under way.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Barton C, Noshchenko A, Patel V, Cain C, Kleck C, Burger E. Risk factors for rod fracture after posterior correction of adult spinal deformity with osteotomy: A retrospective case-series. Scoliosis. 2015. 10: 30-
2. Berjano P, Damilano M, Ismael M, Longo A, Bruno A, Lamartina C. Anterior column realignment (ACR) technique for correction of sagittal imbalance. Eur Spine J. 2015. 24: 451-3
3. Bess S, Harris JE, Turner AW, LaFage V, Smith JS, Shaffrey CI. The effect of posterior polyester tethers on the biomechanics of proximal junctional kyphosis: A finite element analysis. J Neurosurg Spine. 2017. 26: 125-33
4. Bridwell KH, Lewis SJ, Lenke LG, Baldus C, Blanke K. Pedicle subtraction osteotomy for the treatment of fixed sagittal imbalance. J Bone Joint Surgery. 2003. 85-A: 454-63
5. Bridwell KH, Lewis SJ, Edwards C, Lenke LG, Iffrig TM, Berra A. Complications and outcomes of pedicle subtraction osteotomies for fixed sagittal imbalance. Spine. 2003. 28: 2093-101
6. Cahill PJ, Wang W, Asghar J, Booker R, Betz RR, Ramsey C. The use of a transition rod may prevent proximal junctional kyphosis in the thoracic spine after scoliosis surgery: A finite element analysis. Spine. 2012. 37: E687-95
7. Charosky S, Moreno P, Maxy P. Instability and instrumentation failures after a PSO: A finite element analysis. Eur Spine J. 2014. 23: 2340-9
8. Demirkiran G, Theologis AA, Pekmezci M, Ames C, Deviren V. Adult Spinal Deformity Correction with Multi-level Anterior Column Releases: Description of a New Surgical Technique and Literature Review. Clin Spine Surg. 2016. 29: 141-9
9. Deukmedjian AR, Dakwar E, Ahmadian A, Smith DA, Uribe JS. Early outcomes of minimally invasive anterior longitudinal ligament release for correction of sagittal imbalance in patients with adult spinal deformity. ScientificWorldJournal 2012. 2012. p. 789698-
10. Deukmedjian AR, Le TV, Baaj AA, Dakwar E, Smith DA, Uribe JS. Anterior longitudinal ligament release using the minimally invasive lateral retroperitoneal transpsoas approach: A cadaveric feasibility study and report of 4 clinical cases. J Neurosurg Spine. 2012. 17: 530-9
11. Deviren V, Tang JA, Scheer JK, Buckley JM, Pekmezci M, McClellan RT. Construct Rigidity after Fatigue Loading in Pedicle Subtraction Osteotomy with or without Adjacent Interbody Structural Cages. Global Spine J. 2012. 2: 213-20
12. Fujiwara A, Lim TH, An HS, Tanaka N, Jeon CH, Andersson GB. The effect of disc degeneration and facet joint osteoarthritis on the segmental flexibility of the lumbar spine. Spine. 2000. 25: 3036-44
13. Galbusera F, Bassani T, La Barbera L, Ottardi C, Schlager B, Brayda-Bruno M. Planning the Surgical Correction of Spinal Deformities: Toward the Identification of the Biomechanical Principles by Means of Numerical Simulation. Front Bioeng Biotechnol. 2015. 3: 178-
14. Hallager DW, Gehrchen M, Dahl B, Harris JA, Gudipally M, Jenkins S. Use of Supplemental Short Pre-Contoured Accessory Rods and Cobalt Chrome Alloy Posterior Rods Reduces Primary Rod Strain and Range of Motion Across the Pedicle Subtraction Osteotomy Level: An In Vitro Biomechanical Study. Spine. 2016. 41: E388-95
15. Hyun SJ, Lenke LG, Kim YC, Koester LA, Blanke KM. Comparison of standard 2-rod constructs to multiple-rod constructs for fixation across 3-column spinal osteotomies. Spine. 2014. 39: 1899-904
16. Jager ZS, Īnceoğlu S, Palmer D, Akpolat YT, Cheng WK. Preventing Instrumentation Failure in Three-Column Spinal Osteotomy: Biomechanical Analysis of Rod Configuration. Spine Deform. 2016. 4: 3-9
17. Lafage V, Bharucha NJ, Schwab F, Hart RA, Burton D, Boachie-Adjei O. Multicenter validation of a formula predicting postoperative spinopelvic alignment. J Neurosurg Spine. 2012. 16: 15-21
18. Marchi L, Oliveira L, Amaral R, Castro C, Coutinho T, Coutinho E. Anterior elongation as a minimally invasive alternative for sagittal imbalance-a case series. HSS J. 2012. 8: 122-7
19. Melikian R, Yoon ST, Kim JY, Park KY, Yoon C, Hutton W. Sagittal Plane Correction Using the Lateral Transpsoas Approach: A Biomechanical Study on the Effect of Cage Angle and Surgical Technique on Segmental Lordosis. Spine. 2016. 41: E1016-21
20. Murray G, Beckman J, Bach K, Smith DA, Dakwar E, Uribe JS. Complications and neurological deficits following minimally invasive anterior column release for adult spinal deformity: A retrospective study. Eur Spine J. 2015. 24: 397-404
21. Niosi CA, Zhu QA, Wilson DC, Keynan O, Wilson DR, Oxland TR. Biomechanical characterization of the three-dimensional kinematic behaviour of the Dynesys dynamic stabilization system: An in vitro study. Eur Spine J. 2006. 15: 913-22
22. Nguyen TQ, Buckley JM, Ames C, Deviren V. The fatigue life of contoured cobalt chrome posterior spinal fusion rods. Proc Inst Mech Eng H. 2011. 225: 194-8
23. Schilling C, Kruger S, Grupp TM, Duda GN, Blomer W, Rohlmann A. The effect of design parameters of dynamic pedicle screw systems on kinematics and load bearing: An in vitro study. Eur Spine J. 2011. 20: 297-307
24. Schmidt H, Heuer F, Drumm J, Klezl Z, Claes L, Wilke HJ. Application of a calibration method provides more realistic results for a finite element model of a lumbar spinal segment. Clin Biomech. 2007. 22: 377-84
25. Schmoelz W, Huber JF, Nydegger T, Dipl I, Claes L, Wilke HJ. Dynamic stabilization of the lumbar spine and its effects on adjacent segments: An in vitro experiment. J Spinal Disord Tech. 2003. 16: 418-23
26. Uribe JS, Harris JE, Beckman JM, Turner AW, Mundis GM, Akbarnia BA. Finite element analysis of lordosis restoration with anterior longitudinal ligament release and lateral hyperlordotic cage placement. Eur Spine J. 2015. 24: 420-6
27. Uribe JS, Smith DA, Dakwar E, Baaj AA, Mundis GM, Turner AW. Lordosis restoration after anterior longitudinal ligament release and placement of lateral hyperlordotic interbody cages during the minimally invasive lateral transpsoas approach: A radiographic study in cadavers. J Neurosurg Spine. 2012. 17: 476-85
28. Wedemeyer M, Parent S, Mahar A, Odell T, Swimmer T, Newton P. Titanium versus stainless steel for anterior spinal fusions: An analysis of rod stress as a predictor of rod breakage during physiologic loading in a bovine model. Spine. 2007. 32: 42-8