- Department of Neurosurgery, Nagoya City University Graduate School of Medical Sciences, 1 Kawasumi, Mizuho-cho, Mizuho-ku, Nagoya 467-8601, Japan
- Department of Medical informatics and Integrative Medicine, Nagoya City University Graduate School of Medical Sciences, 1 Kawasumi, Mizuho-cho, Mizuho-ku, Nagoya 467-8601, Japan
Correspondence Address:
Hiroyuki Katano
Department of Neurosurgery, Nagoya City University Graduate School of Medical Sciences, 1 Kawasumi, Mizuho-cho, Mizuho-ku, Nagoya 467-8601, Japan
DOI:10.4103/sni.sni_263_17
Copyright: © 2017 Surgical Neurology International This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Hiroyuki Katano, Yusuke Nishikawa, Hiroshi Yamada, Mitsuhito Mase. Calcification in original plaque and restenosis following carotid artery stenting. 20-Nov-2017;8:279
How to cite this URL: Hiroyuki Katano, Yusuke Nishikawa, Hiroshi Yamada, Mitsuhito Mase. Calcification in original plaque and restenosis following carotid artery stenting. 20-Nov-2017;8:279. Available from: http://surgicalneurologyint.com/surgicalint-articles/calcification-in-original-plaque-and-restenosis-following-carotid-artery-stenting/
Abstract
Background:The relationship between calcification in primary plaque and recurrent stenosis after carotid artery stenting (CAS) is not established, but an inverse association with restenosis following carotid endarterectomy (CEA) has been suggested.
Methods:We retrospectively analyzed 75 plaques of 109 consecutive CAS with regard to calcification, using the calcium score and shape, location, and other characteristics of original plaques together with stenting-related factors. CAS was performed in a standard fashion with an embolic protection device. Greater-than-moderate restenosis (≥50%) was assessed by peak systolic velocity (PSV) with duplex ultrasonography (≥130 cm/s, internal/common carotid or distal/proximal PSV ratio ≥2.0).
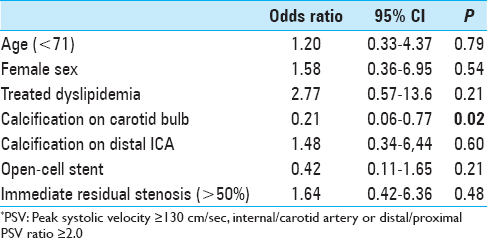
Results:Univariate analysis revealed percentages of dyslipidemia treated with statins (P = 0.03), calcification in distal ICA (P = 0.02), and immediate residual stenosis (P = 0.02) were significantly higher in patients with greater-than-moderate restenosis, whereas calcification in carotid bulb and usage of open-cell stent were rather less frequent (P P = 0.02, respectively). Multivariate logistic regression analysis showed that rarity of calcification in carotid bulb was a sole independent predictor for greater-than-moderate recurrent carotid stenosis 1 year after CAS (OR = 0.21, CI = 0.06–0.77, P = 0.02).
Conclusions:Calcium score was not significantly related to restenosis at 1 year after CAS, as was previously found following CEA, though scarcity of calcification in carotid bulb was suggested as a predictor of in-stent restenosis. Compared to post-CEA restenosis, carotid plaque calcification may be inversely but tenuously associated with recurrent stenosis 1 year post-CAS. No other stenting factors (e.g., stent design, pre-/post-dilation, or protection devices) showed a significant association with recurrent stenosis post-CAS.
Keywords: Calcification, calcium score, carotid artery stenting, carotid stenosis, in-stent restenosis
INTRODUCTION
Recurrent stenosis or in-stent restenosis after carotid artery stenting (CAS) is known as a risk factor of ipsilateral stroke, and this stroke risk should be monitored during patient follow-up.[
A recent research clarified that greater-than-moderate recurrent stenosis after CEA was less frequent in carotid plaques with higher calcium scores, indicating that plaque calcification is inversely related to post-CEA restenosis.[
PATIENTS AND METHODS
Patient population and surgical treatments
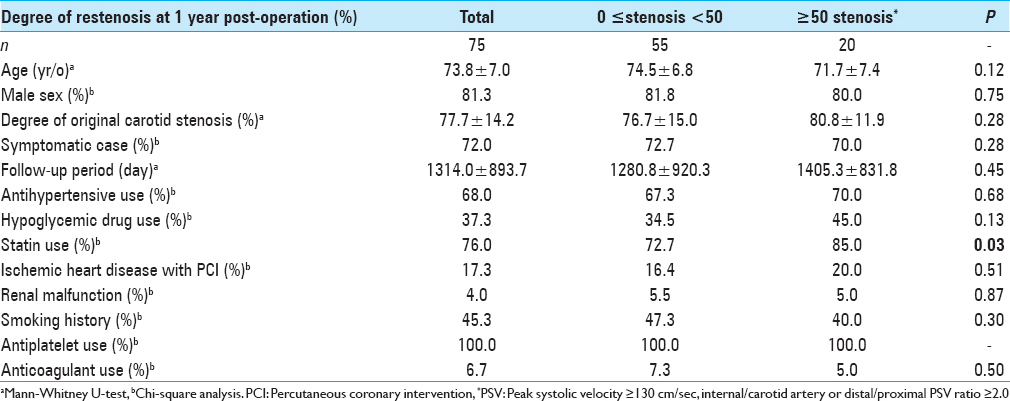
The sample included a total of 109 consecutive cases of CAS performed between February 2005 and December 2015. Fifteen patients with follow-up periods <1 year, seven patients with secondary stenting, 11 patients with missing peak systolic velocity (PSV) data of duplex ultrasonography (DUS) at 1 year, and two patients without computed tomography angiography (CTA) data for renal failure were excluded from the study. A final total of 75 carotid arteries with CAS including two bilateral stentings and four post-CEA cases were enrolled in the study and retrospectively analyzed. The mean follow-up period was 43.3 ± 29.5 months. No transient or permanent focal neurologic symptoms of the contralateral limbs or ipsilateral retina were observed in the patients with postoperative recurrent stenosis. The patient data are summarized in
Surgical indications for the treatment of carotid stenosis adhered to the criteria of the North American Symptomatic Carotid Endarterectomy Trial (NASCET),[
The use of pre-/postdilation and the selection of the types of stents and protection devices were determined at the discretion of the interventionalists. Dual antiplatelet therapy (DAPT) using mainly aspirin (100 mg/day)/clopidogrel (75 mg/day) or cilostazol (200 mg/day) were performed (except in six cases with a clopidogrel/cilostazol combination and two with triple antiplatelet therapy) at ≥5 days before CAS and were continued after the procedure for 3 months; and a lifetime continuation of solely aspirin was prescribed.
The patients’ use(s) of antihypertensives, hypoglycemic drugs, statins, antiplatelets, and anticoagulants were checked. Renal malfunction was defined as serum creatinine >1.5 mg/dl and/or blood urea nitrogen (BUN) >30 mg/dl. History of smoking was defined as a patient who had a pattern of >100 cigarettes/lifetime in the past and/or at the time of recording.
The study design was approved by the local ethics committee, and the ethical guidelines for medical and health research involving human participants issued by the Japanese Health Labor and Welfare Ministry (2014) were strictly observed.
Assessment of the calcium score of the plaques with multidetector row computed tomogaphy
Multidetector row CT (MDCT) angiography was performed preoperatively in all enrolled patients with a 64-detector row CT scanner (SOMATOM Definition: Siemens Medical Solutions, Forchheim, Germany) or a 16-detector row CT scanner (IDT-16: Philips Medical Systems, Best, Netherlands).[
The optimal timing of MDCT angiography acquisition was determined by an automated bolus-timing program. Images were obtained from the aortic arch to the level of the inferior orbits. The image data were transferred to a computer workstation (Ziostation version 1.17; Amin, Tokyo) for image postprocessing, and the calcification of the carotid plaque was preoperatively quantified. The Agatston calcium score[
Assessment of the calcification in carotid plaque, positive remodeling, and stenosis
Assessment of plaque calcification characteristics was preoperatively performed as described.[
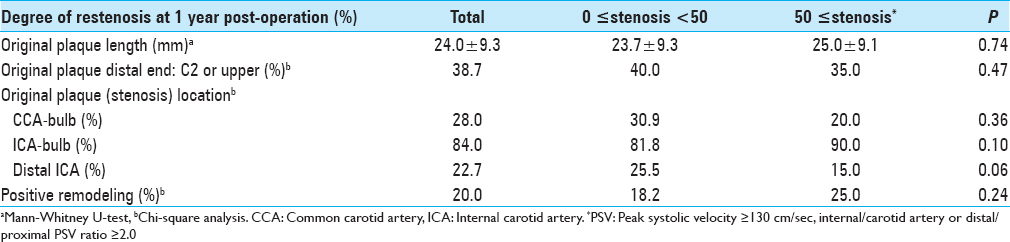
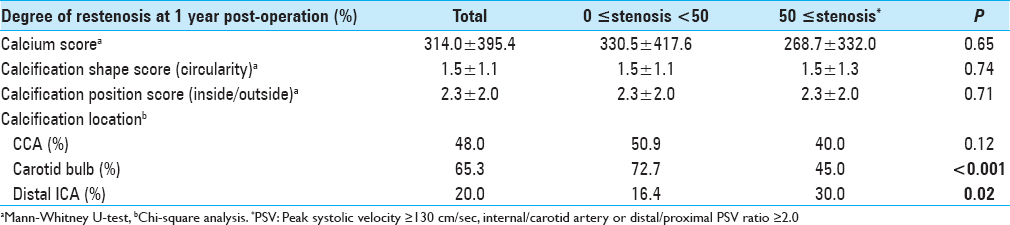
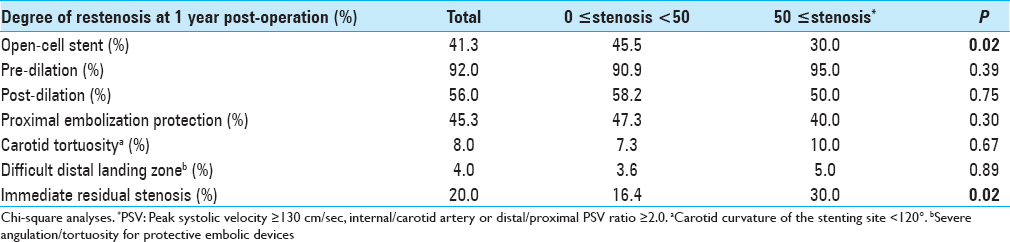
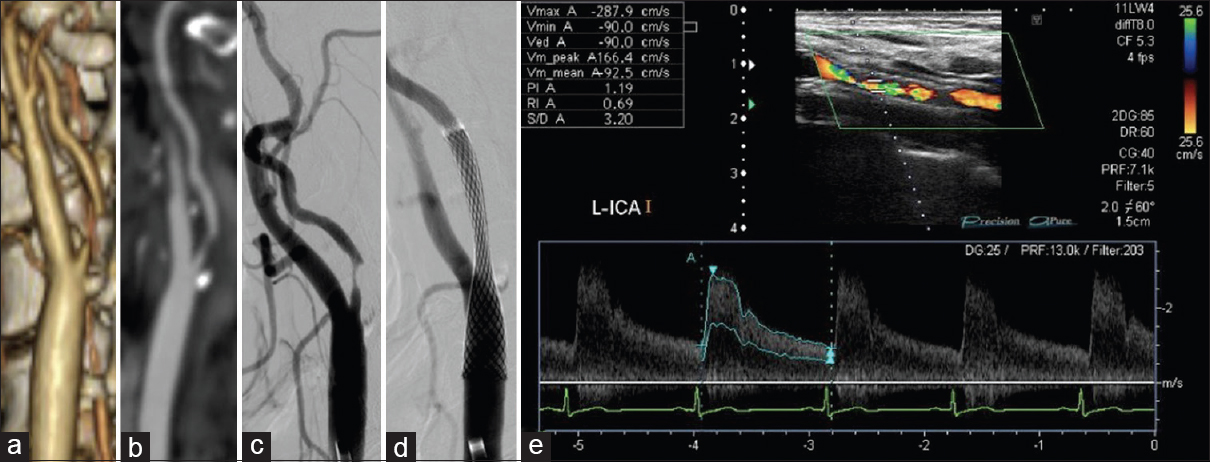
We classified and scored the inner (intimal) and outer (adventitial) position of the calcification in the carotid plaque in the carotid wall based on enhanced CT: 1, inner side in the carotid wall only; 2, inner >outer, 3: inner = outer, 4: inner We determined the degree of pathological findings other than calcification based on histopathological specimens of the extracted plaques stained with hematoxylin-eosin just after the operation and scored as follows: lipid core (0: none, 1: small, 2: medium, 3: large), fibrous tissue (0: none, 1: thin, 2: thick), and intraplaque hemorrhage (0: none, 1: slight, 2: prominent). Positive remodeling was defined as a remodeling ratio (RR) >1.1, where RR = the cross-sectional diameter (CSD) at the point of maximum stenosis in the (ICA)/the reference CSD at the distal ICA.[ The stent types (open-/closed-cell), implementation of pre-dilation/post-dilation, and protection for embolization (distal/proximal) were checked and evaluated. Carotid tortuosity was defined as carotid curvature of the stenting site <120°, and a difficult distal landing zone was determined as severe angulation or tortuosity for protective embolic devices, according to the Buffalo risk assessment scale (BRASS).[ The degree of restenosis after CAS was assessed by PSV on high-resolution ultrasonography, with a linear transducer at 7.5 MHz in the B mode (Aplio, Toshiba Medical Systems, Otawara, Japan). The highest PSV from the treated ICA or CCA was used to identify restenosis. The PSV threshold for predicting ≥50% carotid stenosis was 130 cm/s based on a recent precise study[ All statistical evaluations were performed with Statview ver. 5.0 software (SAS, Cary, NC) and StatMate III (ATMS, Tokyo), and all results are presented as mean ± SD values. The Chi-square test with Yates’ correction and the Mann–Whitney U-test were used for comparisons. For the multivariate analysis, a binary logistic regression model was used. Probability (P) values <0.05 were considered significant. There were no significant differences in basic data concerning age, gender, degree of original carotid stenosis or follow-up period between the groups with no-or-mild stenosis (<50%; n = 55) and greater-than-moderate restenosis (≥50%; n = 20) groups at 1 year postoperation [ We performed a univariate analysis for the above two groups regarding original plaque characteristics. No significant difference was found for the length, height of the distal end, positive remodeling, or location of the initial stenosis [ Concerning the stenting-related factors, the use of an open-cell stent was significantly less frequent in the greater-than-moderate restenosis group, whereas immediate residual stenosis was significantly higher (both P = 0.02) by univariate analysis. No significant differences between the no-or-mild and greater-than-moderate restenosis groups were revealed concerning pre- and post-dilation, the use of proximal embolization protection, carotid tortuosity, and difficult distal landing zone [ The results of the multivariate logistic regression analysis indicated that scarce calcification in the carotid bulb was the only significant independent predictor of post-CAS greater-than-moderate restenosis at 1 year (OR = 0.21, CI = 0.06–0.77, P = 0.02) [ A 84-year-old male with right asymptomatic carotid stenosis. Volume rendering (a), maximum intensity projection (b), images of multidetector row CT and digital subtraction angiography (c), before carotid artery stenting (CAS). Calcification was observed in the carotid bulb and the common carotid artery. (d) The Wallstent® was successfully placed immediately after the procedure. (e) Duplex ultrasonography one year after CAS revealed that the peak systolic velocity was 63.8 cm/s A 81-year-old male with right symptomatic carotid stenosis. Volume rendering (a, b) maximum intensity projection (c), images of multidetector row CT, and digital subtraction angiography (d) before carotid artery stenting (CAS). Calcification was observed in the carotid bulb, the common and distal internal carotid artery. (e) The Wallstent® was successfully placed immediately after the procedure. (f) Duplex ultrasonography one year after CAS revealed that the peak systolic velocity was 91.5 cm/s A 73-year-old male with right symptomatic carotid stenosis. Volume rendering (a), maximum intensity projection (b), images of multidetector row CT and digital subtraction angiography (c), before carotid artery stenting (CAS). Calcification was observed in the common and the distal internal carotid artery. (d) The Wallstent® was successfully placed immediately after the procedure. (e) Duplex ultrasonography one year after CAS revealed that the peak systolic velocity (PSV) was 145.1 cm/s and the PSV ratio for the distal to the proximal portion of the stenosis was 2.0 A 65-year-old male with left symptomatic carotid stenosis. Volume rendering (a), maximum intensity projection (b), images of multidetector row CT and digital subtraction angiography (c), before carotid artery stenting (CAS). Calcification was observed only in the common carotid artery. (d) The Wallstent® was successfully placed immediately after the procedure. (e) Duplex ultrasonography one year after CAS revealed that the peak systolic velocity was 287.9 cm/s and the PSV ratio for the distal to the proximal portion of the stenosis was 6.1 Our study's multivariate regression analysis revealed that scarcity of calcification in the carotid bulb was the sole independent predictor of greater-than-moderate recurrent stenosis after CAS. The calcium score and the calcification circularity, which have been reported to be predictors of restenosis in CEA,[ Prior investigations of restenosis after CAS showed several important and related factors concerning demographics and comorbidity. As a large study, a subanalysis of the CREST trial[ In our present study, we found no significant difference between the no-or-mild restenosis and greater-than-moderate restenosis groups concerning demographics, concomitant conditions, and characteristics of the original plaque and stenosis [Tables Regarding the usage of antiplatelets, several reports indicated a therapeutic effect of cilostazol.[ Regarding the factors related to stent materials, procedures, and the postoperative state, the reported independent predictors of restenosis are immediate post-CAS residual stenosis,[ In our study, though the multivariate analysis revealed no significance, the results of the univariate analysis regarding immediate residual stenosis was in accordance with previous reports,[ Concerning calcification in original plaque, Moon et al.[ On the other hand, Wasser et al.[ Here, we used a calcium score that was an integrated indicator encompassing hardness and volume. We also precisely evaluated the calcification, i.e., its shape, position, and location. However, scarcity of calcification in the carotid bulb was the sole independent predictor by multivariate analysis. The incidence of the greater-than-moderate restenosis that occurred in the area concerning the carotid bulb for plaques without calcification (9/11; 81.8%) was greater than that for plaques with calcification in the same area (33.3%; 3/9). This suggests that calcification was inversely associated with restenosis, which is in line with the findings reported by Misaki et al. A previous analysis concerning restenosis after CEA revealed an apparent inverse relationship with a calcium score and the circularity of calcification,[ The primary limitations of our study are its retrospective character and the small number of cases. We used DUS for the assessment of the degree of in-stent restenosis, whereas the use of PSV to estimate the degree of stenosis varies among researchers.[ Scarcity of calcification in the carotid bulb was the sole predictor of in-stent restenosis 1 year after CAS, and the calcium score was not significantly associated with recurrence, as was observed following CEA. The relationship between carotid calcification and recurrent stenosis 1 year after CAS might be reverse, but it may be more tenuous than that after CEA. None. This research was supported by internal funds only. Nil. There are no conflicts of interest.Assessment of the stenting-related factors
Duplex ultrasonography
Statistical analysis
RESULTS
Baseline characteristics
Original plaque characteristics and factors for stenting
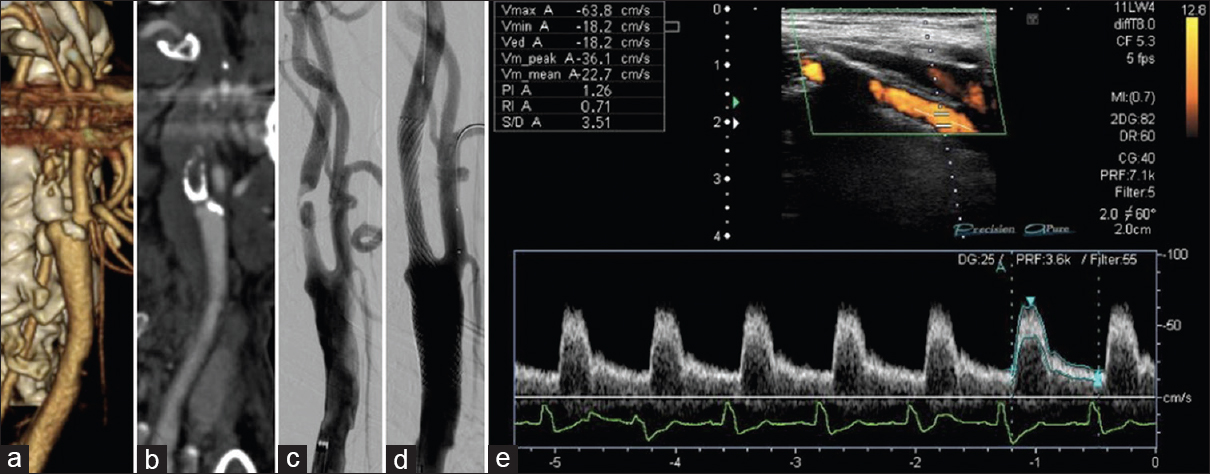
Figure 1
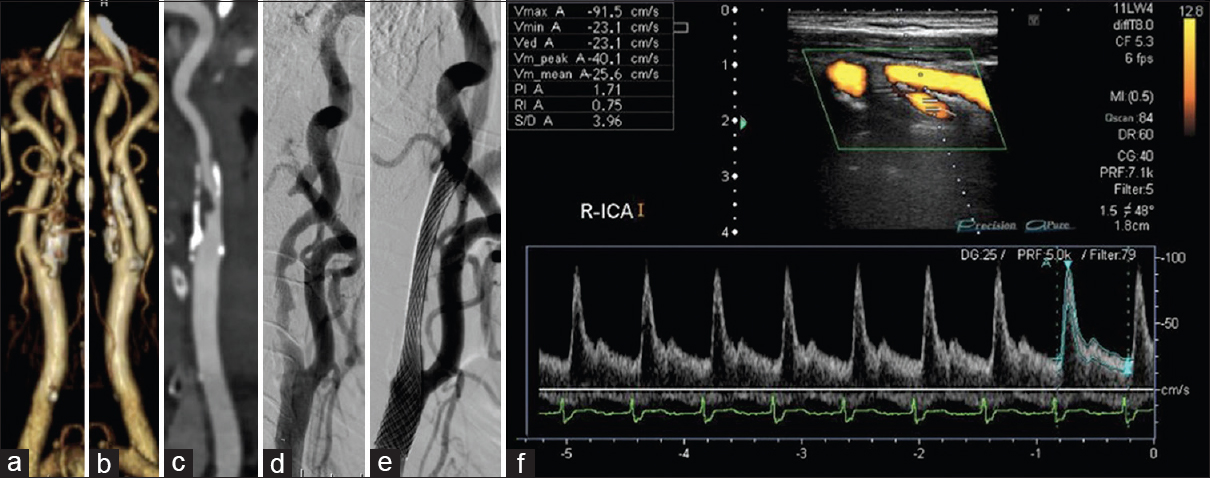
Figure 2
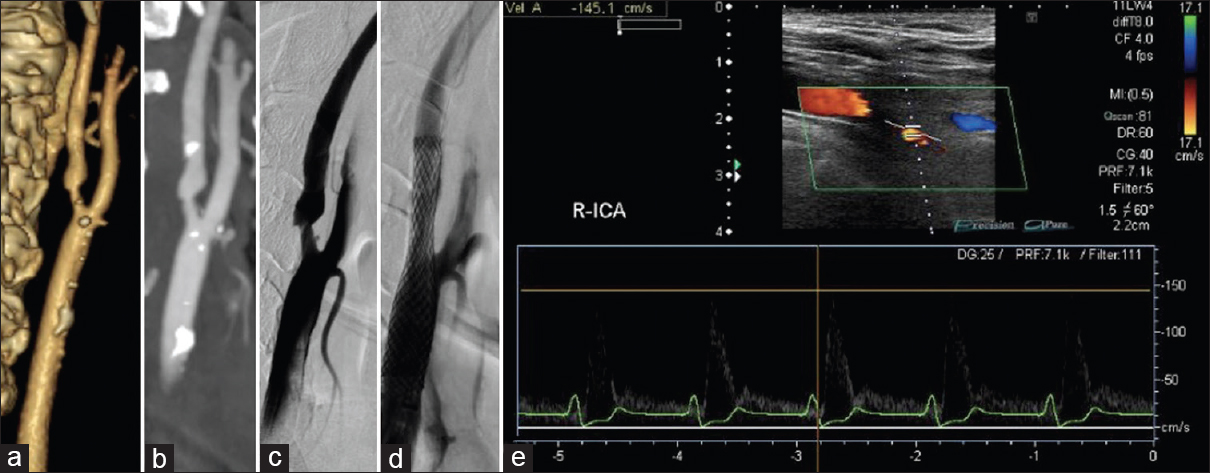
Figure 3
Figure 4
DISCUSSION
Comparison of the present and previous findings regarding restenosis after CAS
Relationship between restenosis and factors related to CAS
Calcification in carotid plaques and restenosis after CAS
Limitations
CONCLUSIONS
Disclosures
Sources of funding
Financial support and sponsorship
Conflicts of interest
Acknowledgments
We thank all of our ward staff for their invaluable clinical contributions.
References
1. . Executive Committee for the Asymptomatic Carotid Atherosclerosis Study. Endarterectomy for asymptomatic carotid artery stenosis. JAMA. 1995. 273: 1421-8
2. AbuRahma AF, Srivastava M, Stone PA, Richmond BK, AbuRahma Z, Jackson W. Effect of statins on early and late clinical outcomes of carotid endarterectomy and the rate of post-carotid endarterectomy restenosis. J Am Coll Surg. 2015. 220: 481-7
3. Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990. 15: 827-32
4. Arquizan C, Trinquart L, Touboul PJ, Long A, Feasson S, Terriat B. EVA-3S Investigators. Restenosis is more frequent after carotid stenting than after endarterectomy: The EVA-3S study. Stroke. 2011. 42: 1015-20
5. Barros P, Felgueiras H, Pinheiro D, Guerra M, Gama V, Veloso M. Restenosis after carotid artery stenting using a specific designed ultrasonographic protocol. J Stroke Cerebrovasc Dis. 2014. 23: 1416-20
6. . Collaborators NASCET. Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis. N Engl J Med. 1991. 325: 445-53
7. Cosottini M, Michelassi MC, Bencivelli W, Lazzarotti G, Picchietti S, Orlandi G. In stent restenosis predictors after carotid artery stenting. Stroke Res Treat. 2010. 2010: 864724-
8. Dai Z, Xue GRestenosis after carotid artery stenting. Vascular. 2017. p.
9. Daou B, Chalouhi N, Starke RM, Dalyai R, Polifka A, Sarkar K. Predictors of restenosis after carotid artery stenting in 241 cases. J Neurointerv Surg. 2016. 8: 677-9
10. de Donato G, Setacci C, Deloose K, Peeters P, Cremonesi A, Bosiers M. Long-term results of carotid artery stenting. J Vasc Surg. 2008. 48: 1431-40
11. Fanous AA, Natarajan SK, Jowdy PK, Dumont TM, Mokin M, Yu J. High-risk factors in symptomatic patients undergoing carotid artery stenting with distal protection: Buffalo Risk Assessment Scale (BRASS). Neurosurgery. 2015. 77: 531-42
12. Gray-Weale AC, Graham JC, Burnett JR, Byrne K, Lusby RJ. Carotid artery atheroma: Comparison of preoperative B-mode ultrasound appearance with carotid endarterectomy specimen pathology. J Cardiovasc Surg (Torino). 1988. 29: 676-81
13. Hashimura N, Mutoh T, Matsuda K, Matsumoto K. Evaluation and management of plaque protrusion or thrombus following carotid artery stenting. Neurol Med Chir (Tokyo). 2015. 55: 149-54
14. Indolfi C, Cioppa A, Stabile E, Di Lorenzo E, Esposito G, Pisani A. Effects of hydroxymethylglutaryl coenzyme A reductase inhibitor simvastatin on smooth muscle cell proliferationin vitro and neointimal formationin vivo after vascular injury. J Am Coll Cardiol. 2000. 35: 214-21
15. Katano H, Mase M, Nishikawa Y, Yamada H, Yamada K. Analysis of recurrent stenosis after carotid endarterectomy featuring primary plaque calcification. Neurosurgery. 2017. 80: 863-70
16. Katano H, Mase M, Nishikawa Y, Yamada K. Calcified carotid plaques show double symptomatic peaks according to Agatston calcium score. J Stroke Cerebrovasc Dis. 2015. 24: 1341-50
17. Katano H, Mase M, Nishikawa Y, Yamada K. Surgical treatment for carotid stenoses with highly calcified plaques. J Stroke Cerebrovasc Dis. 2014. 23: 148-54
18. Katano H, Yamada K. Analysis of calcium in carotid plaques with Agatston scores for appropriate selection of surgical intervention. Stroke. 2007. 38: 3040-4
19. Katano H, Yamada K. Carotid endarterectomy for stenoses of twisted carotid bifurcations. World Neurosurg. 2010. 73: 147-54
20. Kotsugi M, Takayama K, Myouchin K, Wada T, Nakagawa I, Nakagawa H. Carotid artery stenting: Investigation of plaque protrusion incidence and prognosis. JACC Cardiovasc Interv. 2017. 10: 824-83
21. Kwon BJ, Jung C, Sheen SH, Cho JH, Han MH. CT angiography of stented carotid arteries: Comparison with Doppler ultrasonography. J Endovasc Ther. 2007. 14: 489-97
22. Lal BK, Beach KW, Roubin GS, Lutsep HL, Moore WS, Malas MB. CREST Investigators. Restenosis after carotid artery stenting and endarterectomy: A secondary analysis of CREST, a randomised controlled trial. Lancet Neurol. 2012. 11: 755-63
23. Leo HB, Jörg E, Dominick JH, McCabe Joanna D, Featherstone RL, Gaines PA. Long-term risk of carotid restenosis in patients randomly assigned to endovascular treatment or endarterectomy in the Carotid and Vertebral Artery Transluminal Angioplasty Study (CAVATAS): Long-term follow-up of a randomised trial. Lancet Neurol. 2009. 8: 908-17
24. Matsumoto H, Yako R, Masuo O, Hirayama K, Uematsu Y, Nakao N. A Case of In-Stent Neoatherosclerosis 10 Years after Carotid Artery Stent Implantation: Observation with Optical Coherence Tomography and Plaque Histological Findings. Neurol Med Chir (Tokyo). 2014. 54: 139-44
25. Misaki K, Uchiyama N, Mohri M, Hayashi Y, Ueda F, Nakada M. Prediction of carotid artery in-stent restenosis by quantitative assessment of vulnerable plaque using computed tomography. J Neuroradiol. 2016. 43: 18-24
26. Miyazaki Y, Mori T, Iwata T, Aoyagi Y, Tanno Y, Kasakura S. Continuous daily use of cilostazol prevents in-stent restenosis following carotid artery stenting: Serial angiographic investigation of 229 lesions. J Neurointerv Surg. 2016. 8: 471-5
27. Moon K, Albuquerque FC, Levitt MR, Ahmed AS, Kalani MY, McDougall CG. The myth of restenosis after carotid angioplasty and stenting. J Neurointerv Surg. 2016. 8: 1006-10
28. Motoyama S, Kondo T, Sarai M, Sugiura A, Harigaya H, Sato T. Multislice computed tomographic characteristics of coronary lesions in acute coronary syndromes. J Am Coll Cardiol. 2007. 50: 319-26
29. Okahara M, Kiyosue H, Kashiwagi J, Ueda S, Hori Y, Mori H. Small in-stent low density on CT angiography after carotid artery stenting. Interv Neuroradiol. 2008. 14: 41-6
30. Ronchey S, Praquin B, Orrico M, Serrao E, Ciceroni C, Alberti V. Outcomes of 1000 carotid wallstent implantations: Single-center experience. J Endovasc Ther. 2016. 23: 267-74
31. Shankar JJ, Zhang J, dos Santos M, Lesiuk H, Mohan R, Lum C. Factors affecting long-term restenosis after carotid stenting for carotid atherosclerotic disease. Neuroradiology. 2012. 54: 1347-53
32. Shinozaki N, Ogata N, Ikari Y. Plaque protrusion detected by intravascular ultrasound during carotid artery stenting. J Stroke Cerebrovasc Dis. 2014. 23: 2622-5
33. Sun ZS, Zhou SH, Guan X. Impact of blood circulation on reendothelialization, restenosis and atrovastatin's restenosis prevention effects. Int J Cardiol. 2008. 128: 261-8
34. Takigawa T, Matsumaru Y, Hayakawa M, Nemoto S, Matsumura A. Cilostazol reduces restenosis after carotid artery stenting. J Vasc Surg. 2010. 51: 51-6
35. Tokunaga K, Koga M, Yoshimura S, Arihiro S, Suzuki R, Nagatsuka K. Optimal peak systolic velocity thresholds for predicting internal carotid artery stenosis greater than or equal to 50%, 60%, 70%, and 80%. J Stroke Cerebrovasc Dis. 2016. 25: 921-6
36. Wasser K, Karch A, Gröschel S, Witzenhausen J, Gröschel K, Bähr M. Plaque morphology detected with Duplex ultrasound before carotid angioplasty and stenting (CAS) is not a predictor of carotid artery in-stent restenosis, a case control study. BMC Neurol. 2013. 13: 163-
37. Wasser K, Schnaudigel S, Wohlfahrt J, Psychogios MN, Knauth M, Gröschel K. Inflammation and in-stent restenosis: The role of serum markers and stent characteristics in carotid artery stenting. PloS One. 2011. 6: e22683-
38. Yadav JS, Wholey MH, Kuntz RE, Fayad P, Katzen BT, Mishkel GJ. Stenting and Angioplasty with Protection in Patients at High Risk for Endarterectomy Investigators. Protected carotid-artery stenting versus endarterectomy in high-risk patients. N Engl J Med. 2004. 351: 1493-501
39. Yamagami H, Sakai N, Matsumaru Y, Sakai C, Kai Y, Sugiu K. Periprocedural cilostazol treatment and restenosis after carotid artery stenting: The Retrospective Study of In-Stent Restenosis after Carotid Artery Stenting (ReSISteR-CAS). J Stroke Cerebrovasc Dis. 2012. 21: 193-9
40. Zapata-Arriaza E, Moniche F, González A, Bustamante A, Escudero-Martínez I, De la Torre Laviana FJ. Predictors of restenosis following carotid angioplasty and stenting. Stroke. 2016. 47: 2144-7