- Department of Neurosurgery, Faculty of Medicine, University of Tsukuba, Tsukuba, Ibaraki, Japan
- Department of Otolaryngology, Faculty of Medicine, University of Tsukuba, Tsukuba, Ibaraki, Japan
Correspondence Address:
Masahide Matsuda
Department of Neurosurgery, Faculty of Medicine, University of Tsukuba, Tsukuba, Ibaraki, Japan
DOI:10.4103/sni.sni_86_17
Copyright: © 2017 Surgical Neurology International This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Masahide Matsuda, Hiroyoshi Akutsu, Shuho Tanaka, Akira Matsumura. Combined simultaneous transcranial and endoscopic endonasal resection of sphenoorbital meningioma extending into the sphenoid sinus, pterygopalatine fossa, and infratemporal fossa. 10-Aug-2017;8:185
How to cite this URL: Masahide Matsuda, Hiroyoshi Akutsu, Shuho Tanaka, Akira Matsumura. Combined simultaneous transcranial and endoscopic endonasal resection of sphenoorbital meningioma extending into the sphenoid sinus, pterygopalatine fossa, and infratemporal fossa. 10-Aug-2017;8:185. Available from: http://surgicalneurologyint.com/surgicalint-articles/combined-simultaneous-transcranial-and-endoscopic-endonasal-resection-of-sphenoorbital-meningioma-extending-into-the-sphenoid-sinus-pterygopalatine-fossa-and-infratemporal-fossa/
Abstract
Background:Sphenoorbital meningiomas are surgically challenging because of their nature to extend to adjacent structures. Here, we describe a case of recurrent sphenoorbital meningioma extending into the sphenoid sinus, pterygopalatine fossa, and infratemporal fossa, which was resected using combined simultaneous transcranial and endoscopic endonasal approaches.
Case Description:A 62-year-old man who had 15 years earlier undergone partial resection of a left sphenoorbital meningioma presented with a 1-year history of progressive proptosis of the left eye. Magnetic resonance imaging (MRI) showed a Gd-enhancing tumor occupying the left sphenoid wing and orbital lateral wall and extending into extracranial structures such as the sphenoid sinus, pterygopalatine fossa, and infratemporal fossa as well as adjacent structures such as the cavernous sinus and superior orbital fissure (SOF). Based on the MRI findings of tumor extension into the sphenoid sinus with broad continuity, the risk of postoperative cerebrospinal fluid (CSF) leakage through the large defect in the sphenoid sinus was considered high. Subtotal resection using combined simultaneous transzygomatic and endoscopic endonasal approaches was performed, leaving residual tumor in the cavernous sinus and SOF. The large skull base defect between the middle fossa and sphenoid sinus was covered with a free graft of fascia lata from the transcranial side and with a vascularized nasoseptal flap from the endonasal side. No CSF rhinorrhea and no neurological deficits developed postoperatively.
Conclusion:Combined simultaneous transcranial and endoscopic endonasal approaches may become a safe and feasible alternative for sphenoorbital meningioma with a large skull base defect penetrating to the paranasal sinus.
Keywords: Cerebrospinal fluid leakage, combined transcranial and endoscopic endonasal, reconstruction, sphenoorbital meningioma
INTRODUCTION
Sphenoorbital meningioma is a rare entity that accounts for 0.2–9.0% of all intracranial meningiomas.[
CASE REPORT
A 62-year-old man who had 15 years earlier undergone partial resection for left sphenoorbital meningioma in another hospital presented with a 1-year history of progressive proptosis of the left eye. He was referred to our hospital because magnetic resonance imaging (MRI) and computed tomography (CT) revealed a hyperostotic tumor of the left sphenoid wing with orbital and middle fossa extension. On admission, ophthalmic examination showed slight proptosis of the left eye, but visual acuities, visual field areas, eye movements, and pupillary light reflexes remained normal. Neurological examination showed no neurological deficits. The results of laboratory examinations, including thyroid function, were essentially normal. CT performed in our hospital revealed hypertrophy of the left sphenoid wing, orbital lateral wall, and floor of the middle fossa [Figure
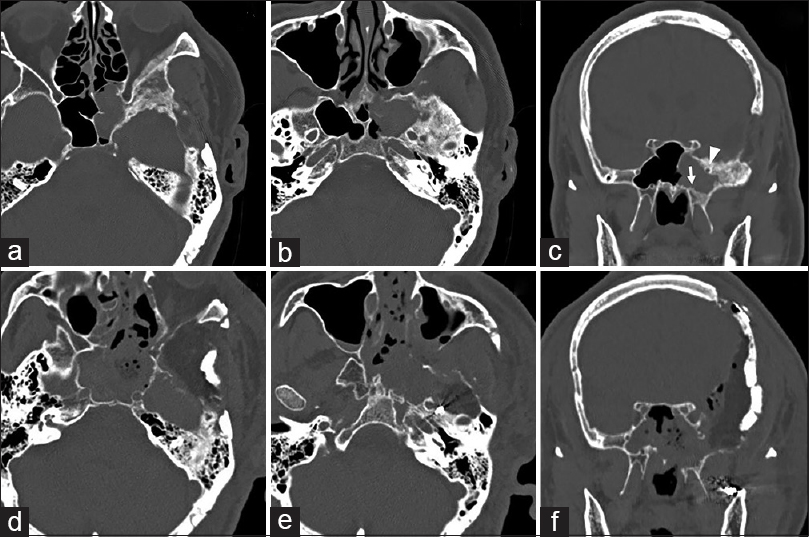
Figure 1
Pre- and postoperative computed tomography (CT). Preoperative CT in the bone window in the axial plane (a and b) demonstrating hypertrophy of the left sphenoid wing, orbital lateral wall, and floor of the middle fossa. Pre-operative CT in the bone window in the coronal plane (c) showing development of the lateral recess of the sphenoid sinus beyond the sphenoid body into the greater wing. Postoperative CT in the bone window (d–f) showing extensively drilled sphenoid wing, orbital lateral wall, floor of the middle fossa, ethmoid sinus, and sphenoid sinus. Arrow, vidian canal; arrowhead, foramen rotundum
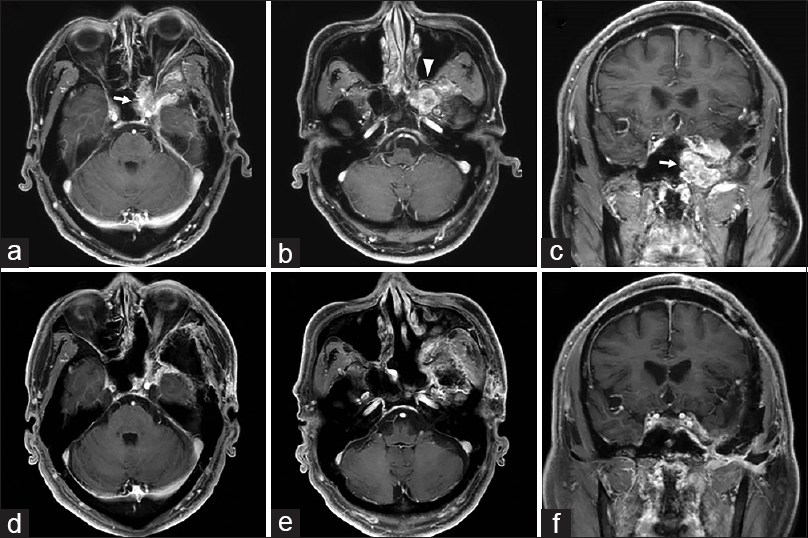
Figure 2
Pre- and postoperative magnetic resonance imaging. Pre-operative axial T1-weighted imaging with gadolinium contrast (a and b) and sagittal T1-weighted imaging with gadolinium contrast (c) demonstrating a homogeneously enhancing tumor extending into extracranial structures as well as adjacent structures. Postoperative axial T1-weighted imaging with gadolinium contrast (d and e) and sagittal T1-weighted imaging with gadolinium contrast (f) demonstrating residual tumor only in the cavernous sinus and superior orbital fissure. Arrows, tumor extending into the sphenoid sinus; arrowhead, tumor extending into the pterygopalatine fossa
Surgical procedure
Under general anesthesia, the patient was placed supine with the head fixed in a Mayfield clamp and rotated 30° to the right. To achieve both transcranial and endoscopic endonasal approaches, two surgical teams worked simultaneously. The transcranial surgeon stood at the head end of the patient and the endonasal surgeon stood on the right side of the patient.
Transcranial approach
The frontotemporal craniotomy made 15 years earlier was used and an additional zygomatic osteotomy was created to approach the tumor. The thickened hyperostotic sphenoid wing and lateral wall of the orbit were completely drilled out and removed. After cutting of the meningo-orbital band followed by peeling of the dura propria of the middle fossa from the lateral wall of the cavernous sinus, further drilling around the SOF and floor of the middle fossa was performed. Following identification of the maxillary and mandibular nerves, the floor of the middle fossa around the foramen rotundum and foramen ovale was drilled until the pterygopalatine fossa and infratemporal fossa were exposed. During these procedures, the tumor mass extending into the sphenoid sinus was exposed and the sphenoid sinus was then widely opened by resection of the tumor mass [
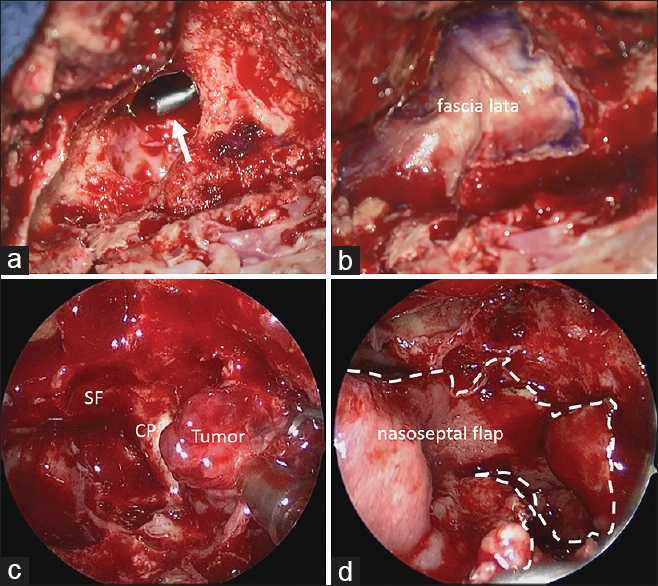
Figure 4
Intraoperative photographs. (a) Intraoperative photograph from the transcranial side showing the large bone defect of the sphenoid sinus after tumor resection. Arrow indicates suction from the endonasal side. (b) Intraoperative photograph demonstrating repair of the defect with a free graft of fascia lata. (c) Intraoperative photograph from the endonasal side showing tumor extending into the sphenoid sinus. SF, sellar floor; CP, carotid prominence. (d) Intraoperative photograph demonstrating repair of the defect with a pedicled nasoseptal flap. Dotted line indicates the margin of the nasoseptal flap
Endoscopic endonasal approach
A rigid high-definition endoscope (Karl Storz, Tuttlingen, Germany) was used in a standard endonasal approach to expose the sphenoid sinus. A pedicled NSF was then prepared while maintaining the vascular supply from the posterior septal branch of the sphenopalatine artery.[
DISCUSSION
Infiltration to surrounding structures is one of the most striking features of sphenoorbital meningioma. Accordingly, radical resection of sphenoorbital meningioma is still challenging, with a significant risk of complications. The main structures limiting radical resection are the cavernous sinus and SOF, in which tumors directly infiltrate cranial nerves and extend along connective tissue planes in between these nerves.[
CSF leakage is one of the most frequent and potentially threatening complication after resection of skull base tumors. To avoid CSF leaks, use of vascularized flaps such as temporoparietal fascial flap, pericranial flap, or galeopericranial flap is highly recommended to reconstruct the defect, particularly in cases with a large skull base defect penetrating to the paranasal sinus.[
The combination of simultaneous transcranial and transsphenoidal approaches was first described by Loyo et al. in 1984 for the resection of extremely large pituitary tumors.[
CONCLUSION
Combined simultaneous transcranial and endoscopic endonasal approaches may offer a safe and feasible alternative for resecting sphenoorbital meningiomas with extracranial extension. In particular, when a tumor penetrates and extends into the sphenoid sinus with broad continuity, this technique contributes to not only facilitating aggressive resection of tumor infiltration into the skull base bone and extension into the paranasal sinus, but also preventing postoperative CSF leakage using a vascularized NSF. It should be emphasized, however, that application of combined approaches requires careful patient selection and tailored modification depending on the experience of the surgical teams.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Attia M, Patel KS, Kandasamy J, Stieg PE, Spinelli HM, Riina HA. Combined cranionasal surgery for spheno-orbital meningiomas invading the paranasal sinuses, pterygopalatine, and infratemporal fossa. World Neurosurg. 2013. 80: e367-73
2. Bloss HG, Proescholdt MA, Mayer C, Schreyer AG, Brawanski A. Growth pattern analysis of sphenoid wing meningiomas. Acta Neurochir. 2010. 152: 99-103
3. Cophignon J, Lucena J, Clay C, Marchac D. Limits to radical treatment of spheno-orbital meningiomas. Acta Neurochir Suppl (Wien). 1979. 28: 375-80
4. D’Ambrosio AL, Syed ON, Grobelny BT, Freda PU, Wardlaw S, Bruce JN. Simultaneous above and below approach to giant pituitary adenomas: Surgical strategies and long-term follow-up. Pituitary. 2009. 12: 217-25
5. Hadad G, Bassagasteguy L, Carrau RL, Mataza JC, Kassam A, Snyderman CH. A novel reconstructive technique after endoscopic expanded endonasal approaches: Vascular pedicle nasoseptal flap. Laryngoscope. 2006. 116: 1882-6
6. Hara T, Akutsu H, Yamamoto T, Tanaka S, Takano S, Ishikawa E. Cranial Base Repair Using Suturing Technique Combined with a Mucosal Flap for Cerebrospinal Fluid Leakage During Endoscopic Endonasal Surgery. World Neurosurg. 2015. 84: 1887-93
7. Hirsch O. Successful closure of cere brospinal fluid rhinorrhea by endonasal surgery. AMA Arch Otolaryngol. 1952. 56: 1-12
8. Hofstetter CP, Singh A, Anand VK, Kacker A, Schwartz TH. The endoscopic, endonasal, transmaxillary transpterygoid approach to the pterygopalatine fossa, infratemporal fossa, petrous apex, and the Meckel cave. J Neurosurg. 2010. 113: 967-74
9. Kassam AB, Thomas A, Carrau RL, Snyderman CH, Vescan A, Prevedello D. Endoscopic reconstruction of the cranial base using a pedicled nasoseptal flap. Neurosurgery. 2008. 63: ONS44-52
10. Leung GK, Law HY, Hung KN, Fan YW, Lui WM. Combined simultaneous transcranial and transsphenoidal resection of large-to-giant pituitary adenomas. Acta Neurochir. 2011. 153: 1401-8
11. Loyo M, Kleriga E, Mateos H, de Leo R, Delgado A. Combined supra-infrasellar approach for large pituitary tumors. Neurosurgery. 1984. 14: 485-8
12. Maroon JC, Kennerdell JS, Vidovich DV, Abla A, Sternau L. Recurrent spheno-orbital meningioma. J Neurosurg. 1994. 80: 202-8
13. Neligan PC, Mulholland S, Irish J, Gullane PJ, Boyd JB, Gentili F. Flap selection in cranial base reconstruction. Plast Reconstr Surg. 1996. 98: 1159-66
14. Nishioka H, Hara T, Usui M, Fukuhara N, Yamada S. Simultaneous combined supra-infrasellar approach for giant/large multilobulated pituitary adenomas. World Neurosurg. 2012. 77: 533-9
15. Ojha BK, Husain M, Rastogi M, Chandra A, Chugh A, Husain N. Combined trans-sphenoidal and simultaneous trans-ventricular-endoscopic decompression of a giant pituitary adenoma: Case report. Acta Neurochir. 2009. 151: 843-7
16. Pompili A, Derome PJ, Visot A, Guiot G. Hyperostosing meningiomas of the sphenoid ridge-clinical features, surgical therapy, and long-term observations: Review of 49 cases. Surg Neurol. 1982. 17: 411-6
17. Romano A, Chibbaro S, Marsella M, Oretti G, Spiriev T, Iaccarino C. Combined endoscopic transsphenoidal-transventricular approach for resection of a giant pituitary macroadenoma. World Neurosurg. 2010. 74: 161-4
18. Sandalcioglu IE, Gasser T, Mohr C, Stolke D, Wiedemayer H. Spheno-orbital meningiomas: Interdisciplinary surgical approach, resectability and long-term results. J Craniomaxillofac Surg. 2005. 33: 260-6
19. Scarone P, Leclerq D, Heran F, Robert G. Long-term results with exophthalmos in a surgical series of 30 sphenoorbital meningiomas. Clinical article. J Neurosurg. 2009. 111: 1069-77
20. Shrivastava RK, Sen C, Costantino PD, Della Rocca R. Sphenoorbital meningiomas: Surgical limitations and lessons learned in their long-term management. J Neurosurg. 2005. 103: 491-7
21. Yessenow RS, McCabe BF. The osteo-mucoperiosteal flap in repair of cerebrospinal fluid rhinorrhea: A 20-year experience. Otolaryngol Head Neck Surg. 1989. 101: 555-8