- Department of Neurosurgery, Faculty of Medicine, Fukuoka University, Fukuoka, Japan
- Department of Emergency and Critical Care Medicine, Faculty of Medicine, Fukuoka University, Fukuoka, Japan
Correspondence Address:
Kenji Yagi
Department of Neurosurgery, Faculty of Medicine, Fukuoka University, Fukuoka, Japan
DOI:10.4103/sni.sni_346_17
Copyright: © 2018 Surgical Neurology International This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.How to cite this article: Masani Nonaka, Kenji Yagi, Hiroshi Abe, Koichi Miki, Takashi Morishita, Mitsutoshi Iwaasa, Tooru Inoue. Endoscopic surgery via a combined frontal and suboccipital approach for cerebellar hemorrhage. 05-Apr-2018;9:68
How to cite this URL: Masani Nonaka, Kenji Yagi, Hiroshi Abe, Koichi Miki, Takashi Morishita, Mitsutoshi Iwaasa, Tooru Inoue. Endoscopic surgery via a combined frontal and suboccipital approach for cerebellar hemorrhage. 05-Apr-2018;9:68. Available from: http://surgicalneurologyint.com/surgicalint-articles/endoscopic-surgery-via-a-combined-frontal-and-suboccipital-approach-for-cerebellar-hemorrhage/
Abstract
Background:Spontaneous cerebellar hemorrhages (CHs), which frequently require surgical intervention, are life-threatening and can be complicated by intraventricular hemorrhages (IVHs) and obstructive hydrocephalus. Commonly, endoscopic surgery is performed to remove CHs via a suboccipital approach (SA) alone. At our institution, when patients exhibited supratentorial IVH-associated hydrocephalus, we used a combined frontal and suboccipital approach (CA) to evacuate both CHs and supratentorial IVHs. The present study retrospectively evaluated the effectiveness and safety of this CA, as no prior studies examining this approach currently exist.
Methods:Twenty-six patients with spontaneous CH were surgically treated at our hospital from April 2009 to March 2016. Twenty-two patients who could independently perform activities of daily living before the onset underwent endoscopic surgery to evacuate the CHs; among these, 13 patients underwent the SA alone, while nine underwent the CA. We assessed and compared the patients’ baseline characteristics, surgical results, and prognosis at 1 month after the intervention between the SA and CA groups.
Results:Patients who underwent the CA had significantly poorer consciousness before the surgery owing to IVH extension and obstructive hydrocephalus. However, the surgical results and prognosis at 1 month were not significantly different between the two approaches. The CH-associated IVHs were successfully removed with the CA and resulted in shorter external ventricular drainage (EVD) placement durations.
Conclusion:Endoscopic surgery performed via the CA appeared to neutralize the deteriorating effects of CH-associated IVHs. Surgical strategies employing the CA may have the potential to improve the prognosis of patients with CH.
Keywords: Cerebellar hemorrhage, endoscopic surgery, intraventricular hemorrhage, hydrocephalus
INTRODUCTION
Spontaneous cerebellar hemorrhages (CHs) account for approximately 10% of all spontaneous intracerebral hemorrhage (sICH) cases and are commonly attributed to hypertension.[
Prior studies have shown that CHs are frequently complicated by subsequent obstructive hydrocephalus owing to ventricle compression and intraventricular hematomas (IVHs).[
It has been reported that endoscopic evacuation is effective in providing a favorable prognosis to patients with an sICH or IVH.[
In our institution, CHs and/or CH-associated IVHs are removed using minimally invasive endoscopic surgery. More specifically, we use the suboccipital approach (SA) to remove CHs and/or IVHs in the fourth ventricle, while we employ a combined suboccipital and frontal approach (CA) to endoscopically remove massive IVHs in the supratentorial region. In the present study, we evaluated the efficacy and safety of using the new CA surgical procedure.
MATERIALS AND METHODS
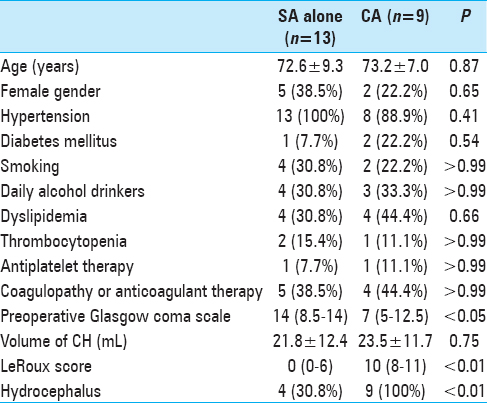
In total, we reviewed the medical records of 41 patients with spontaneous CH who were treated at our hospital from April 2009 to March 2016. The protocol for this retrospective study was approved by the ethics committee at our institution. Patients with traumatic or secondary hematoma associated with aneurysm, vascular malformations, neoplasm, or evident hemorrhagic infarction were not included in this study. Of the 41 patients, 26 were treated surgically. In one patient, the parenchymal CH was not significant but a massive IVH and acute hydrocephalus were present. Therefore, surgery was only performed via a frontal burr hole for the IVH and hydrocephalus but not for the parenchymal CH. The other 25 patients had significant parenchymal CHs, which were surgically removed by endoscopic surgery via an SA or CA. After excluding three patients with moderate to poor pre-hemorrhage activities of daily living [modified Rankin Scale (mRS) score ≥3], the surgical outcomes were compared between the remaining patients treated with the SA or CA. The demographic data and clinical characteristics of the 22 included patients are shown in
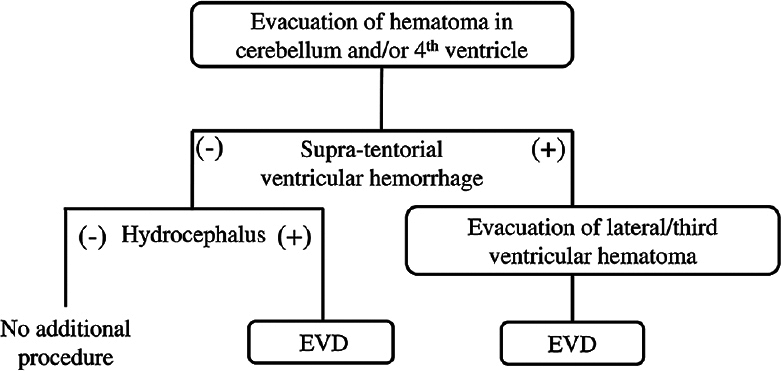
The surgical indications were a large CH (diameter ≥3 cm), brain stem compression, obstructive hydrocephalus, and/or a reduced level of consciousness. Our operative strategy [reviewed in
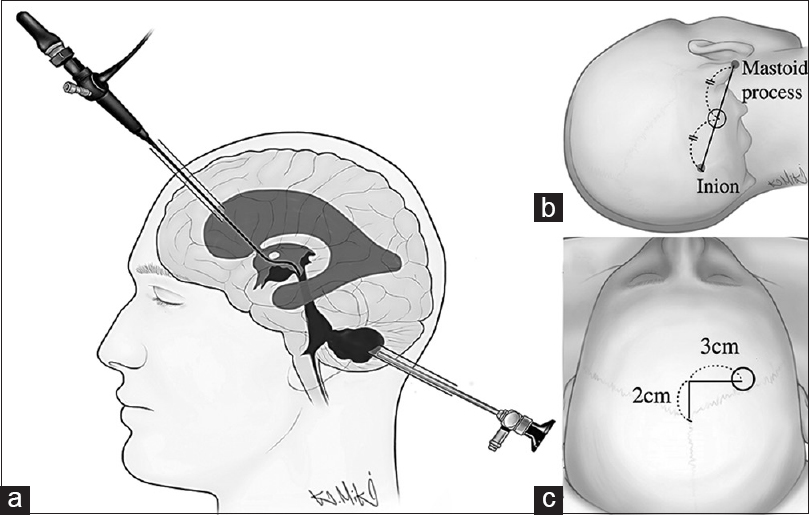
Endoscopic surgery was performed under general anesthesia in a dry field. Patients were placed in the supine position with their ipsilateral shoulders elevated by a pillow and with their heads mildly flexed and rotated toward the contralateral side. A 3-cm linear incision and 1.4-cm diameter burr hole were made at the point closest to the CH cavity, around the midpoint between the ipsilateral mastoid tip and inion [
The CA was applied for patients exhibiting supratentorial IVH-causing hydrocephalus and is described as follows. After CH evacuation via the SA, patients were repositioned supine with their heads in a neutral position, giving the surgeon good access to the ventricles. A frontal skin incision and burr hole were then made 3 cm lateral and 2 cm anterior to bregma on the side where a larger proportion of the hematoma was located in the lateral ventricle [
Patients with hypertension were defined as those with a systolic blood pressure of ≥140 mmHg and/or a diastolic blood pressure of ≥90 mmHg, as well as patients who were on antihypertensive drugs. Patients with dyslipidemia were defined as those with a low-density lipoprotein cholesterol level (≥140 mg/dL) and/or a high-density lipoprotein cholesterol level (<40 mg/dL), as well as patients taking lipid-lowering drugs. Thrombocytopenia was defined as a blood cell count of 80 × 103/μL, and coagulopathy was defined as an activated partial thromboplastin time ≥40 s or a prothrombin time-international normalized ratio >1.4. Patients were classified as diabetic if their level of glycosylated hemoglobin A1C exceeded 6.5% or if they were being treated with insulin and/or oral hypoglycemic drugs. Patients were considered to be current smokers if they had smoked tobacco within the past year and to be daily drinkers if they consumed alcoholic beverages every day. The Glasgow Coma Scale (GCS) score at admission, pre- and postoperative hematoma volumes and extension of the IVH, duration of EVD, surgical complications including bleeding and surgical site infection, and mRS score at 1 month after surgical intervention were all recorded. If the patient transferred to another hospital or clinic, the mRS score was evaluated by interviewing the patient via telephone or based on a report from another institution. The cerebellar hematoma volume was calculated using the simplified equation 1/2A × B × C, where A is the maximum width measured, B is the length, and C is the height.[
All statistical analyses were performed with SPSS version 24 software (IBM Corp., Tokyo, Japan). For the univariate analyses, we used Fisher's exact tests, Student's t-tests, or Mann-Whitney U-tests. Differences were considered significant at P < 0.05. Numerical data are expressed as the mean ± standard deviation or median (interquartile range).
RESULTS
In nine of the 22 patients, the CA was applied. Of these, eight patients underwent CA surgeries from the frontal and suboccipital sides immediately, while in one patient, the CA was applied after an initial surgery using the SA because a supratentorial IVH–causing occlusive hydrocephalus was identified on a CT image after CH evacuation. The remaining 13 patients were treated by SA alone. Comparisons of the patients’ demographic data and clinical characteristics are shown in
Preoperative consciousness was significantly reduced in patients who underwent surgery with the CA compared to those treated by SA alone. In addition, the LeRoux score and frequency of hydrocephalus were significantly higher in the CA-treated patients than in the SA-treated patients. Patient ages and CH volumes were not different between the two treatment groups. The patients treated with the CA were considered to have more severe brain damage neurologically and radiographically.
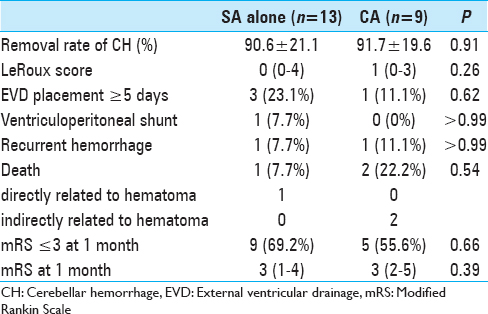
The surgical outcomes are shown in
Owing to non-communicative hydrocephalus, an EVD device was placed in four of the 13 patients (30.8%) treated by SA alone and in all nine patients who underwent treatment via the CA. Although all patients who underwent the CA had remarkable IVH and hydrocephalus, long-duration EVD (≥5 days) was only needed in one patient who had a recurrent CH postoperatively. In the other eight patients, EVD was only required for a few days, as the IVH was successfully removed via the CA. In three of the patients who underwent the SA alone, long-duration EVD was required for the following reasons: high preoperative LeRoux score (8 and 14) and IVH-associated third ventricle occlusion in two patients, and fourth ventricle compression due to an insufficiently removed CH in one patient. Of the three patients who underwent long-duration EVD, one received a ventriculoperitoneal shunt (VPS) for hydrocephalus owing to an IVH-induced wall adhesion in the third ventricle.
Although the data for patients who underwent CA showed that this group had an increased preoperative rate of poorer consciousness, severe IVHs, and frequent hydrocephalus, no remarkable differences in prognosis were noted between this group of patients and the patients who underwent treatment via the SA alone. More specifically, no significant differences in modified Rankin Scale (mRS) scores were identified between the SA-treated and CA-treated groups. Nine (69.2%) of the 13 patients who underwent the SA alone and five (55.6%) of the nine patients who underwent the CA were almost able to independently perform activities of daily living (mRS ≤3).
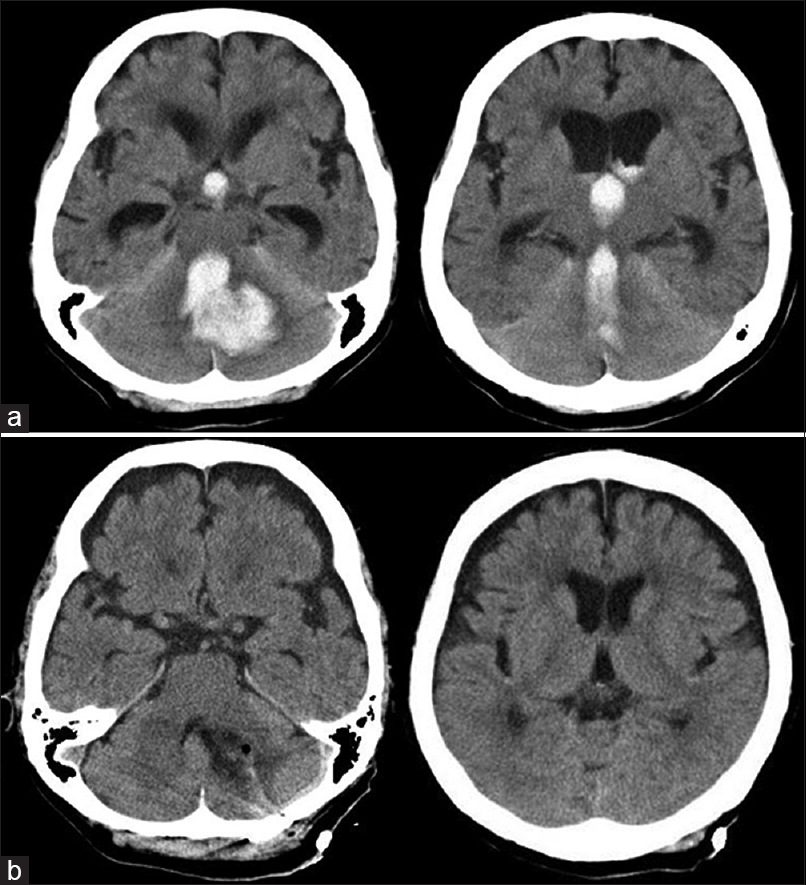
Illustrative case
A 78-year-old female presented with sudden deterioration of consciousness (GCS scores: E1V2M6). Brain CT showed a CH, which extended through the fourth ventricle to the supratentorial ventricle, resulting in non-communicating acute hydrocephalus [
DISCUSSION
The present findings showed that endoscopic evacuation of IVHs by the CA is effective for immediately improving CH-associated obstructive hydrocephalus. Moreover, the CA was associated with a shorter duration of EVD device placement, and thus may have the potential to reduce frequency of intracranial infection or VPS dependency and to facilitate early ambulation.
As previously mentioned, CHs are often accompanied by IVHs, which are associated with ventricular dysfunction, intracranial hypertension, reduced cerebral perfusion, and secondary cerebral injuries.[
The most preferred treatment method for controlling obstructive hydrocephalus is EVD, and the application of EVD devices often results in a decreased IVH-related mortality rate.[
Based on our findings, endoscopic surgery by CA is useful to treat CH patients with supratentorially extended IVHs. Removal of an IVH associated with a CH was found to shorten the duration of EVD, thereby inhibiting VPS dependence and EVD-related infections. Although all nine patients who underwent the CA had IVH-associated obstructive hydrocephalus, only one patient needed long-duration EVD owing to recurrent bleeding. Moreover, none of the patients in the CA group were VPS-dependent. In contrast, three patients that underwent SA alone needed long-duration EVD and one case resulted in VPS dependence. In our retrospective assessments of the preoperative CT scans of the three abovementioned patients, the CA may have been a better strategy for two of the patients because of the presence of supratentorial IVHs.
In terms of prognostic factors and outcomes, previous studies have used the following parameters to evaluate CH prognosis: hematoma size, location of the hematoma relative to the midline, presence of IVH or hydrocephalus, decreased level of consciousness, and neuroradiological or clinical signs of brainstem damage.[
CONCLUSION
Our results support that endoscopic evacuation via a CA might be useful for treating patients with IVH associated–hydrocephalus. Surgical strategies employing this CA have the potential to improve the prognosis of patients with CHs. However, further prospective studies are needed to confirm our findings.
Declaration of patient consent
The authors certify that they have obtained an appropriate consent form of the presented patient. In the form the patient has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
Dr. K. Yagi received grant support from the Japan Society for the Promotion of Science and AOSpine. Dr. T. Morishita received grant support from the Japan Society for the Promotion of Science, St. Luke Life Science Institute, Nakatomi Foundation, Takeda Science Foundation, Uehara Memorial Foundation, and Central Research Institute of Fukuoka University. He also received honoraria from Boston Scientific and Medtronic as a consultant. Dr. T. Inoue received grant support from the Clinical Research Promotion Foundation Japan and Japan Agency for Medical Research and Development.
References
1. Chen CC, Liu CL, Tung YN, Lee HC, Chuang HC, Lin SZ. Endoscopic surgery for intraventricular hemorrhage (IVH) caused by thalamic hemorrhage: Comparisons of endoscopic surgery and external ventricular drainage (EVD) surgery. World Neurosurg. 2011. 75: 264-8
2. Donauer E, Loew F, Faubert C, Alesch F, Schaan M. Prognostic factors in the treatment of cerebellar haemorrhage. Acta Neurochir (Wien). 1994. 131: 59-66
3. Gaberel T, Magheru C, Emery E. Management of non-traumatic intraventricular hemorrhage. Neurosurg Rev. 2012. 35: 485-94
4. Hwang BY, Bruce SS, Appelboom G, Piazza MA, Carpenter AM, Gigante PR. Evaluation of intraventricular hemorrhage assessment methods for predicting outcome following intracerebral hemorrhage. J Neurosurg. 2012. 116: 185-92
5. Kirollos RW, Tyagi AK, Ross SA, van Hille PT, Marks PV. Management of spontaneous cerebellar hematomas: A prospective treatment protocol. Neurosurgery. 2001. 49: 1378-86
6. Komatsu F, Komatsu M, Wakuta N, Oshiro S, Tsugu H, Iwaasa M. Comparison of clinical outcomes of intraventricular hematoma between neuroendoscopic removal and extraventricular drainage. Neurol Med Chir (Tokyo). 2010. 50: 972-6
7. Li Y, Zhang H, Wang X, She L, Yan Z, Zhang N. Neuroendoscopic surgery versus external ventricular drainage alone or with intraventricular fibrinolysis for intraventricular hemorrhage secondary to spontaneous supratentorial hemorrhage: A systematic review and meta-analysis. PLoS One. 2013. 8: e80599-
8. Ochalski P, Chivukula S, Shin S, Prevedello D, Engh J. Outcomes after endoscopic port surgery for spontaneous intracerebral hematomas. J Neurol Surg A Cent Eur Neurosurg. 2014. 75: 195-205
9. Orakcioglu B, Beynon C, Bosel J, Stock C, Unterberg AW. Minimally invasive endoscopic surgery for treatment of spontaneous intracerebral hematomas: A single-center analysis. Neurocrit Care. 2014. 21: 407-16
10. Rosenow F, Hojer C, Meyer-Lohmann C, Hilgers RD, Muhlhofer H, Kleindienst A. Spontaneous intracerebral hemorrhage. Prognostic factors in 896 cases. Acta Neurol Scand. 1997. 96: 174-82
11. Shin D, Woo HJ, Park J. Spontaneous cerebellar hemorrhage with the fourth ventricular hemorrhage: Risk factors associated with ventriculoperitoneal shunt. J Korean Neurosurg Soc. 2012. 52: 320-4
12. Tamaki T, Kitamura T, Node Y, Teramoto A. Paramedian suboccipital mini-craniectomy for evacuation of spontaneous cerebellar hemorrhage. Neurol Med Chir (Tokyo). 2004. 44: 578-82
13. Tsitsopoulos PP, Tobieson L, Enblad P, Marklund N. Prognostic factors and long-term outcome following surgical treatment of 76 patients with spontaneous cerebellar haematoma. Acta Neurochir (Wien). 2012. 154: 1189-95
14. Witsch J, Neugebauer H, Zweckberger K, Juttler E. Primary cerebellar haemorrhage: Complications, treatment and outcome. Clin Neurol Neurosurg. 2013. 115: 863-9
15. Yamamoto T, Nakao Y, Mori K, Maeda M. Endoscopic hematoma evacuation for hypertensive cerebellar hemorrhage. Minim Invasive Neurosurg. 2006. 49: 173-8
16. Yokosuka K, Hirano K, Miyamoto T, Toi H, Matsuzaki K, Matsubara S. Endoscppic evacuation for cerebellar hemorrhage. Surg Cereb Stroke. 2011. 39: 193-7
17. Young WB, Lee KP, Pessin MS, Kwan ES, Rand WM, Caplan LR. Prognostic significance of ventricular blood in supratentorial hemorrhage: A volumetric study. Neurology. 1990. 40: 616-9
18. Zhang J, Wang L, Xiong Z, Han Q, Du Q, Sun S. A treatment option for severe cerebellar hemorrhage with ventricular extension in elderly patients: Intraventricular fibrinolysis. J Neurol. 2014. 261: 324-9