- Unit of Neurosurgery, “Di Venere” City Hospital, Bari, Italy
- Department of Neurosurgery, University “Magna Graecia”, Catanzaro, Italy
Correspondence Address:
Domenico Murrone
Unit of Neurosurgery, “Di Venere” City Hospital, Bari, Italy
DOI:10.4103/sni.sni_398_17
Copyright: © 2018 Surgical Neurology International This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Domenico Murrone, Bruno Romanelli, Nicola Montemurro, Domenico Chirchiglia, Aldo Ierardi. Falcine meningioma in Von Hippel–Lindau disease: An unusual association. 14-Feb-2018;9:37
How to cite this URL: Domenico Murrone, Bruno Romanelli, Nicola Montemurro, Domenico Chirchiglia, Aldo Ierardi. Falcine meningioma in Von Hippel–Lindau disease: An unusual association. 14-Feb-2018;9:37. Available from: http://surgicalneurologyint.com/surgicalint-articles/falcine-meningioma-in-von-hippel-lindau-disease-an-unusual-association/
Abstract
Background:Von Hippel–Lindau (VHL) disease is an autosomal dominant condition characterized by formation of multiple benign and malignant tumors. In this disease supratentorial lesions are rare and no falcine meningioma has been previously reported. Differential diagnosis is very difficult and the histopathological examination is the definitive method for diagnosis.
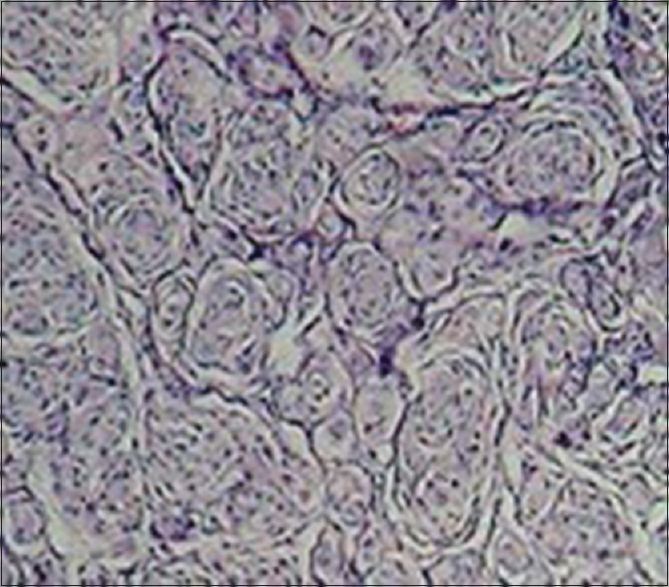
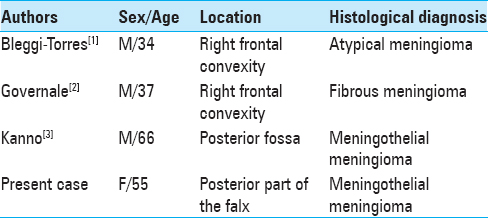
Case Description:A patient with VHL underwent a suboccipital craniotomy for removal of cerebellar hemangioblastoma and after 2 years magnetic resonance imaging (MRI) showed an iperintense solid mass located at posterior part of the falx. Histological diagnosis revealed meningioma.
Conclusion:The only case in the literature of falcine meningioma in a patient with Von Hippel–Lindau disease, discovered during radiological follow-up, is described and a surgical management is proposed.
Keywords: Falcine meningioma, hemangioblastoma, Von Hippel-Lindau disease
INTRODUCTION
Von Hippel–Lindau (VHL) disease is a rare condition caused by genetic mutations on chromosome 3,[
CASE REPORT
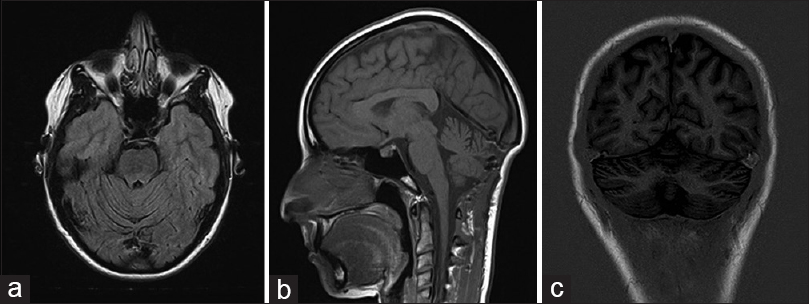
A 55-year-old Caucasian female patient underwent a suboccipital craniotomy for removal of left cerebellar hemisphere HB. Abdominal computed tomography revealed a pheochromocytoma that was excised. Genetic analysis showed the presence of a VHL gene mutation and an evaluation of the family history demonstrated VHL disease in two of the patient's siblings. Thus this patient's condition had been diagnosed as VHL disease. Follow-up magnetic resonance imaging (MRI) performed 1 year after the first operation had shown no evidence of recurrence or abnormal findings in the supratentorial region [
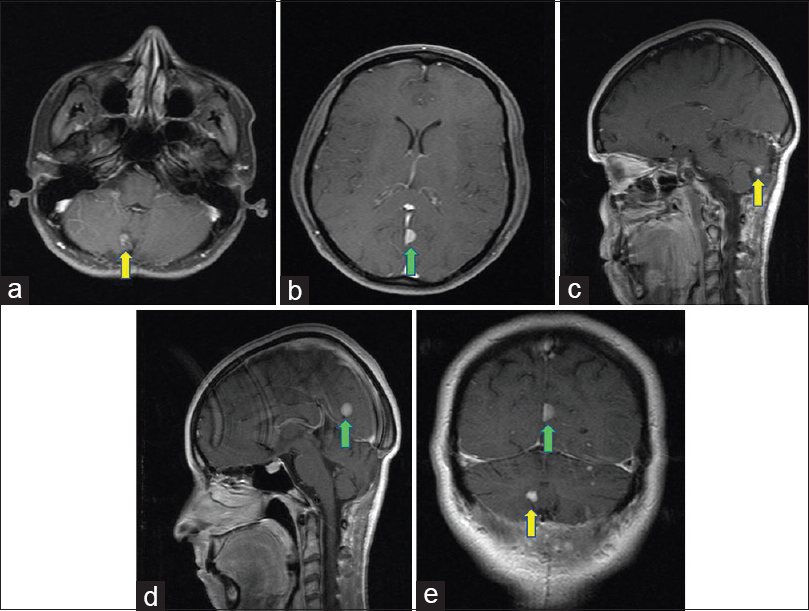
Figure 2
Two years postoperative axial (a and b), sagittal (c and d), a coronal (e) T1-weighted MR images with gadolinium detecting a solid mass with strong enhancement in the right cerebellar hemisphere (yellow arrow) and an hyperintense extra-axial solid mass located at posterior part of the falx (green arrow)
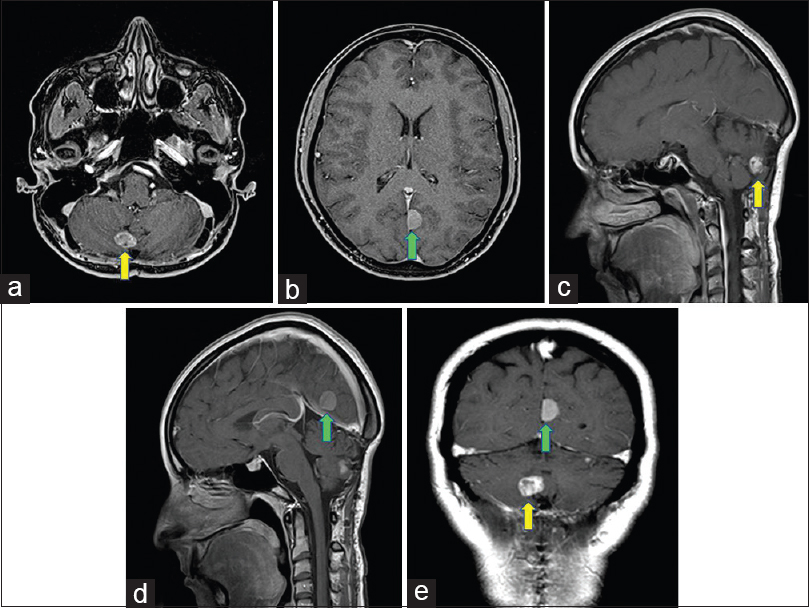
Figure 3
Four years postoperative axial (a and b), sagittal (c and d), a coronal (e) T1-weighted MR images with gadolinium demonstrating further slow growth of both lesions described in
DISCUSSION
Melmon and Rosen[
CONCLUSIONS
We report a rare case of asymptomatic slow-growing meningioma located at the third posterior of the falx in a patient with VHL, detected 2 years after surgical resection of a cerebellar HB, in which no surgical or radiotherapical treatment has been previously performed. In VHL, supratentorial meningeal masses are extremely rare and no other case of falcine involvement has been previously reported. Pathological and immunohistochemical techniques are helpful for differential diagnosis. Regular follow-up is mandatory and surgical resection is the definitive treatment for these lesions.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Binderup ML, Bisgaard ML, Harbud V, Moller HU, Gimsing S, Friis-Hansen L.editors. Von Hippel-Lindau disease (vHL). National clinical guideline for diagnosis and surveillance in Denmark. Dan Med J. 2013. 60: B4763-
2. Bleggi-Torres LF, De Noranha L, Fillus Neto J, Telles JE, Madalozzo LE. Von Hippel-Lindau's disease: Report of three cases and review of the literature. Arq Neuropsiquiatr. 1995. 53: 782-8
3. Chittiboina P, Lonser RR. Von Hippel-Lindau disease. Handb Clin Neurol. 2015. 132: 139-56
4. Governale LS, Vortmeyer AO, Zhuang Z, Oldfield EH. Fibrous meningioma in a patient with von Hippel-Lindau disease: A genetic analysis. J Neurosurg. 2001. 95: 1045-9
5. Ishwar S, Taniguchi RM, Vogel FS. Multiple supratentorial hemangioblastomas. Case study and ultrastructural characteristics. J Neurosurg. 1971. 35: 396-405
6. Kaelin WG. Molecular basis of the VHL hereditary cancer syndrome. Nat Rev Cancer. 2002. 2: 673-82
7. Kaloostian P, Taylor C. Supratentorial dural-based haemangioblastoma in a native American patient without Von Hippel Lindau Syndrome. J Surg Case Rep. 2012. 2012: 11-
8. Kanno H, Yamamoto I, Yoshida M, Kitamura H. Meningioma showing VHL gene inactivation in a patient with von Hippel-Lindau disease. Neurology. 2003. 60: 1197-9
9. Kim H, Park IS, Jo KW. Meningeal supratentorial hemangioblastoma in a patient with von Hippel-Lindau disease mimicking angioblastic meningioma. J Korean Neurosurg Soc. 2013. 54: 415-9
10. Lamiell JM. Army clinical investigation of von Hippel-Lindau disease, 1977-2000. Mil Med. 2001. 166: 839-42
11. Lee KR, Kishore PR, Wulfsberg E, Kepes JJ. Supratentorial leptomeningeal hemangioblastoma. Neurology. 1978. 28: 727-30
12. Lonser RR, Glenn GM, Walther M, Chew EY, Libutti SK, Linehan WM. von Hippel-Lindau disease. Lancet. 2003. 361: 2059-67
13. Maddock IR, Moran A, Maher ER, Teare MD, Norman A, Payne SJ. A genetic register for von Hippel-Lindau disease. J Med Genet. 1996. 33: 120-7
14. Melmon KL, Rosen SW. Lindau's disease. Review of the literature and study of a large kindred. Am J Med. 1964. 36: 595-617
15. Murali R, Jones WI, Ma Wyatt J. A 57-year-old man with a dural-based parietal lobe tumor. Brain Pathol. 2007. 17: 460-3
16. Ning XH, Zhang N, Li T, Wu PJ, Wang X, Li XY. Telomere shortening is associated with genetic anticipation in Chinese Von Hippel-Lindau disease families. Cancer Res. 2014. 74: 3802-9
17. Takei H, Bhattacharjee MB, Rivera A, Dancer Y, Powell SZ. New immunohistochemical markers in the evaluation of central nervous system tumors: A review of 7 selected adult and pediatric brain tumors. Arch Pathol Lab Med. 2007. 131: 234-41






Giovanni
Posted March 14, 2018, 6:59 am
Dear authors,
This is a very interesting work, however some details are missing. Could you specify which pVHL mutation you found in your “Case report”?
Thk