- Department of Neurological Surgery, Montefiore Medical Center, New York, USA
- Department of Pathology, Montefiore Medical Center, New York, USA
- Department of Pathology, Memorial Sloan Kettering Cancer Center, New York, USA
Correspondence Address:
Neil Haranhalli
Department of Neurological Surgery, Montefiore Medical Center, New York, USA
DOI:10.4103/sni.sni_331_16
Copyright: © 2017 Surgical Neurology International This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Neil Haranhalli, Adam E. Ammar, Karen M. Weidenheim, Marc K. Rosenblum, David J. Altschul. Hemorrhagic intracranial follicular dendritic cell sarcoma: A case report. 10-Oct-2017;8:248
How to cite this URL: Neil Haranhalli, Adam E. Ammar, Karen M. Weidenheim, Marc K. Rosenblum, David J. Altschul. Hemorrhagic intracranial follicular dendritic cell sarcoma: A case report. 10-Oct-2017;8:248. Available from: http://surgicalneurologyint.com/surgicalint-articles/hemorrhagic-intracranial-follicular-dendritic-cell-sarcoma-a-case-report/
Abstract
Background:Follicular dendritic cell (FDC) sarcoma is an extremely rare neoplasm, which has only been reported once in the literature with an intracranial occurrence. Neither hemorrhagic presentation of an intracranial instance of FDC sarcoma nor its rapid recurrence has yet been published in the literature.
Case Description:We report the case of a 61-year-old female who presented with confusion and headaches secondary to a right frontal hemorrhagic lesion, and her subsequent presentations for recurrence of the lesion and finding of a new intracranial lesion. Immunohistopathologic analysis confirmed the diagnosis based on immunoreactivity for clusterin and CD 35.
Conclusion:As demonstrated in this case report, the presentation and progression of primary intracranial follicular dendritic cell sarcoma can often be misleading, and consideration for this rare entity should be made in cases of hemorrhagic dural-based lesions without a primary source of malignancy.
Keywords: CD 35, clusterin, follicular dendritic cell sarcoma
BACKGROUND
Follicular dendritic cell (FDC) sarcoma is an extremely rare neoplasm that represents only 0.4% of all soft tissue sarcomas.[
CASE REPORT
Initial presentation
History
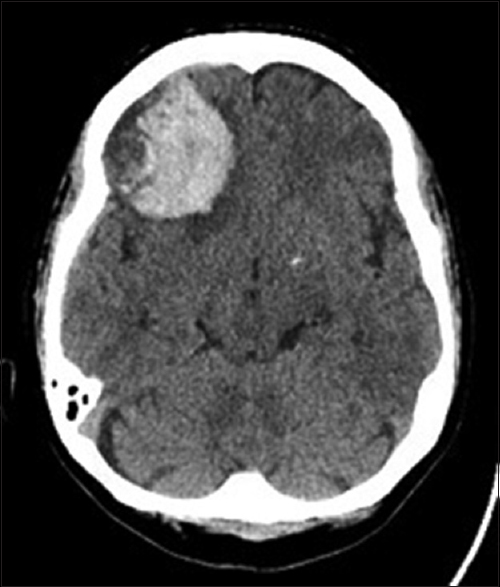
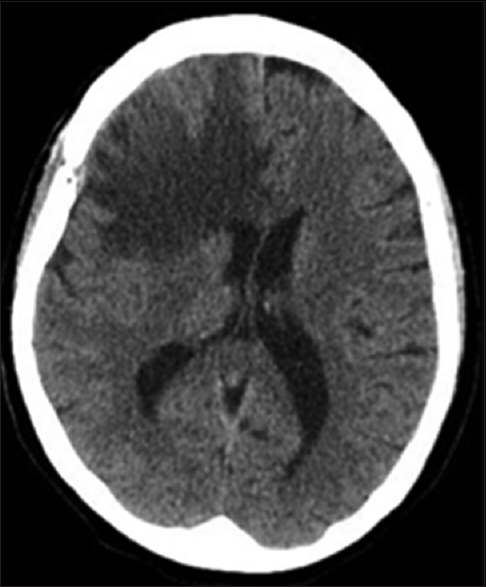
Patient BC is a 61-year-old female with a past medical history of diabetes mellitus and hyperlipidemia who initially presented to the emergency room in June 2015 for periods of confusion and memory problems over the prior few weeks. Upon further questioning, the patient also reported having intermittent, diffuse headaches over the prior week. On initial examination, she was grossly neurologically intact and did not exhibit any noticeable short or long-term memory deficits. A noncontrast head computed tomography (CT) scan revealed a 4 cm right frontal hematoma with associated edema and sulcal effacement along with 3 mm of right-to-left midline shift [
Imaging findings
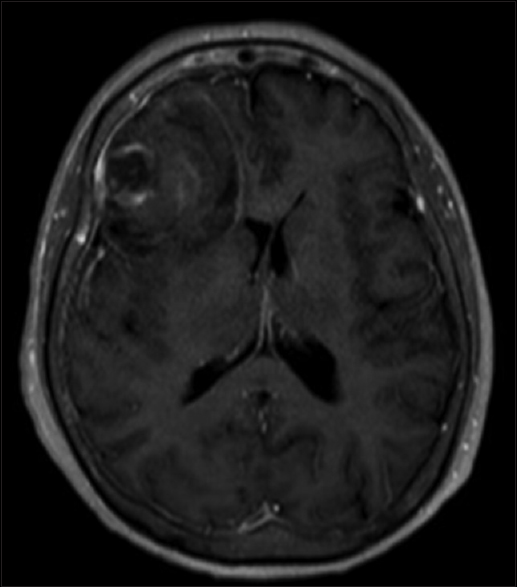
Magnetic resonance imaging (MRI) of the brain with and without contrast done on the night of admission revealed stable acute hemorrhage in the right frontal lobe with underlying dural-based lesions in the right frontal lobe including a dominant hemorrhagic lesion measuring up to 2.4 cm [
Hospital course
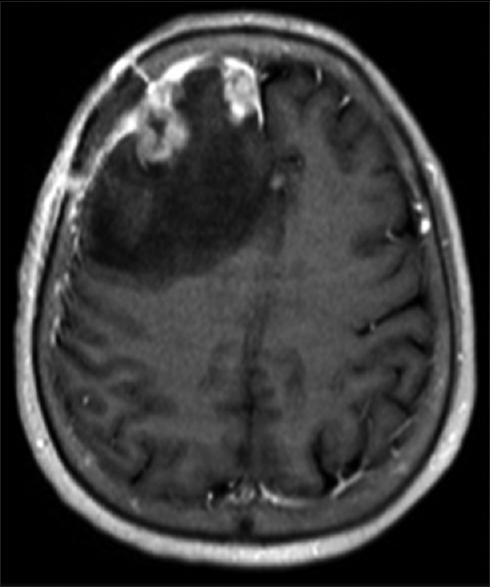
The patient was started on dexamethasone and underwent imaging studies to fully evaluate her intracranial hemorrhage. Evaluation for a primary malignancy source found no other sites of disease. The decision was made to proceed with surgical resection, and the patient underwent a right frontal craniotomy with resection of the dural-based lesion with no adverse events. Postoperatively, the steroids were tapered and she was transferred to an acute rehabilitation facility. Postoperative MRI brain with and without contrast demonstrated enhancement along the margins of the resection cavity, compatible with a resolving residual hematoma [
Histopathology
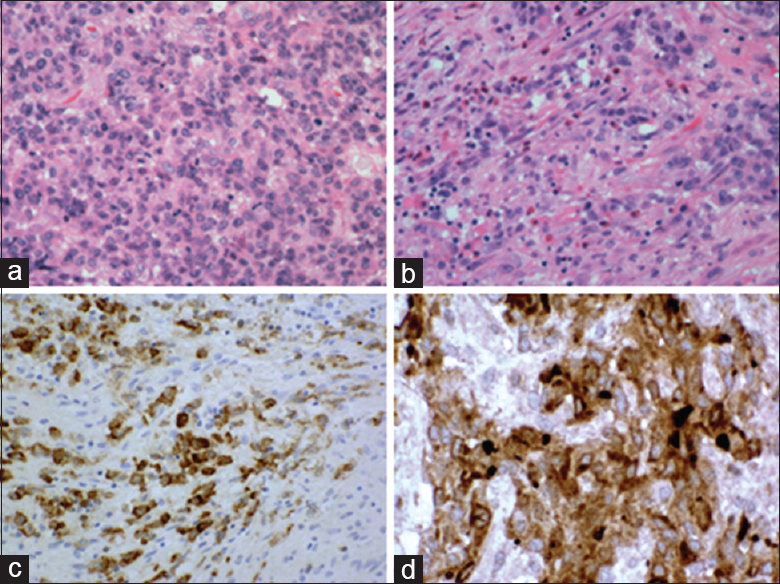
Histologic examination revealed a mitotically active neoplasm with spindled and epithelioid components, conspicuous infiltration by chronic inflammatory elements, including many lymphocytes, and with associated hemorrhagic necrosis. On immunohistochemical staining, there was focal labeling for CD35 and more diffuse expression of clusterin [
Figure 4
Histopathology demonstration of resected mass at initial presentation. (a) Much of the neoplasm consisted of uniform cells with pink cytoplasm and vesicular nuclei having single small nucleoli and many mitoses. H and E, ×375. (b) In other areas, the tumor was infiltrated by an inflammatory cell reaction rich in eosinophils. H and E, ×375. (c and d) Immunohistochemical marker studies were unrevealing except for CD35 and clusterin. Photography courtesy of Dr. M. Rosenblum, Memorial Sloan Kettering Cancer Center, New York, NY. X375
Second presentation
History
The patient was sent to the emergency room from the clinic in August 2015 for confusion, urinary frequency, and hyperglycemia. She was still complaining of difficulty with her memory, as well as feeling drowsy, which was slightly worse than previous. She also complained of an episode of feeling shaking and confusion, which she was concerned was a seizure. On neurologic examination, she was once again grossly intact. She had not yet started radiotherapy or any adjuvant therapy for her brain tumor. CTH was performed in the ED, which demonstrated vasogenic edema in the right frontal lobe and 9 mm of the midline shift [
Imaging findings
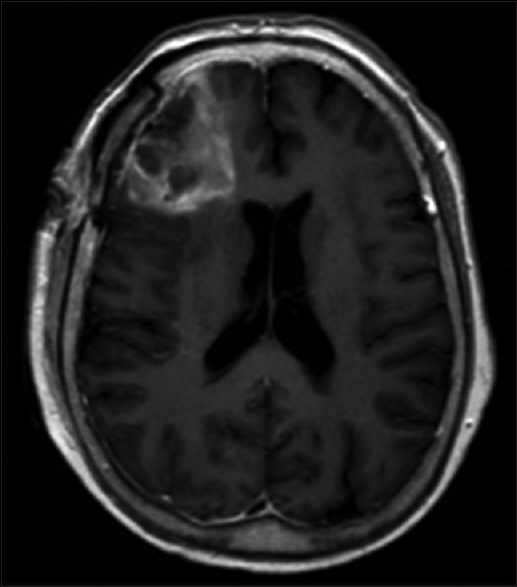
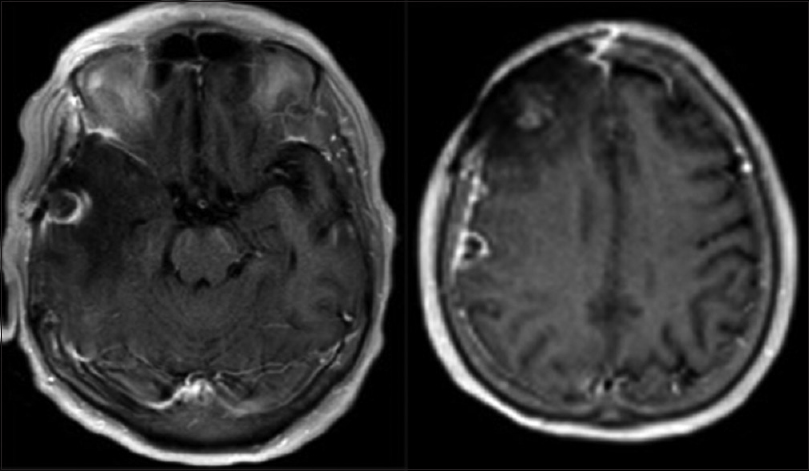
MRI brain with and without contrast on admission demonstrated increasing enhancement and edema in the region of the right frontal resection cavity, including a new 1.4 cm nodular focus of enhancement along the falx cerebri [
Hospital course
The patient's Keppra dose was increased and she was restarted on dexamethasone. She underwent repeat right frontal craniotomy for resection of both the frontal and midline dural-based masses with no adverse events. The patient received her first dose of fractionated frameless stereotactic radiosurgery, and was discharged on a dexamethasone taper and instructions to follow-up for further radiotherapy.
Histopathology
The specimen was similar to that of the previous resection, consisting of dura attached to granulation tissue, with associated large neoplastic pleomorphic cells in nests and areas of necrosis. The neoplasm once again expressed vimentin. Immunohistochemical staining was positive for p53 and CK7. Stains for AE1/3, BCL2, CAM5.2, CD21/31/34/35, Clusterin, CK20, and Synaptophysin were negative.
Third presentation
History
The patient presented again to the emergency room in December 2015 with dizziness and urinary urgency. She was found to have a UTI, but CTH on presentation demonstrated a new 1.5 cm hyperdense lesion in the right middle cranial fossa and a 5 mm lesion in the right high frontal convexity, with associated worsened vasogenic edema and midline shift, concerning for recurrent neoplasm. The patient once again complained of gradually worsening headaches and confusion, with difficulty in memory.
Imaging findings
MRI brain with and without contrast demonstrated increased size of the midfrontal enhancing lesion, as well as a new area of enhancement in the right anterior temporal lobe, with associated edema and mass effect [
Hospital course
The patient was started on high dose steroids and antiepileptics and improved clinically. After discussion with the patient, the decision was made to not pursue further neurosurgical intervention. The patient was initiated on bevacizumab and irinotecan.
Clinical course
Based on the patient and family wishes, the patient was made DNR/DNI prior to discharge with the decision for no further aggressive or invasive measures. She succumbed to her illness and expired in April 2016.
DISCUSSION
We have reported here the original case of this 61-year-old woman presenting with a right frontal hemorrhagic lesion, which diagnosed as FDC sarcoma because of its patterns of tumor cell growth, association with an inflammatory infiltrate, and expression of clusterin and CD35 in the absence of evidence for any other diagnosis. We suspect that given its radiographic similarities with other entities, such as meningioma, intracranial FDC sarcoma may in fact be an underdiagnosed entity and has been mistaken for an inflammatory pseudotumor,[
While the differential diagnosis for solitary intracranial lesions is often headed by entities such as meningioma, gliomas, or metastases of unknown primary, this case report highlights key features of FDC sarcoma which may guide the evaluation of this entity. First, clinical presentation of hemorrhage in meningioma is relatively rare (1–3%).[
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Chan AC, Chan KW, Chan JK, Au WY, Ho WK, Ng WM. Development of follicular dendritic cell sarcoma in hyaline-vascular Castleman's disease of the nasopharynx: Tracing its evolution by sequential biopsies. Histopathology. 2001. 38: 510-8
2. Chan JK, Fletcher CD, Nayler SJ, Cooper K. Follicular dendritic cell sarcoma. Clinicopathologic analysis of 17 cases suggesting a malignant potential higher than currently recognized. Cancer. 1997. 79: 294-313
3. Cheuk W, Chan JK, Shek TW, Chang JH, Tsou MH, Yuen NW. Inflammatory pseudotumor-like follicular dendritic cell tumor: A distinctive low-grade malignant intra-abdominal neoplasm with consistent Epstein-Barr virus association. Am J Surg Pathol. 2001. 25: 721-31
4. Grogg KL, Macon WR, Kurtin PJ, Nascimento AG. A survey of clusterin and fascin expression in sarcomas and spindle cell neoplasms: Strong clusterin immunostaining is highly specific for follicular dendritic cell tumor. Mod Pathol. 2005. 18: 260-6
5. Hasselblatt M, Sepehrnia A, von Falkenhausen M, Paulus W. Intracranial follicular dendritic cell sarcoma. Case report. J Neurosurg. 2003. 99: 1089-90
6. Hornick JL.editorsPractical Soft Tissue Pathology: A Diagnostic Approach. Philadelphia: Elsevier Saunders; 2013. p. 257-261
7. Hutter G, Hofer S, Tzankov A, Kothbauer KF. Intracranial Interdigitating Dendritic Cell Sarcoma:First Case Report. Neurosurgery. 2015. 77: E979-83
8. Kim JH, Gwak HS, Hong EK, Bang CW, Lee SH, Yoo H. A case of benign meningioma presented with subdural hemorrhage. Brain Tumor Res Treat. 2015. 3: 30-3
9. Kosco MH, Gray D. Signals involved in germinal center reactions. Immunol Rev. 1992. 126: 63-76
10. Monda L, Warnke R, Rosai J. A primary lymph node malignancy with features suggestive of dendritic reticulum cell differentiation. A report of 4 cases. Am J Pathol. 1986. 122: 562-72
11. Perez-Ordonez B, Rosai J. Follicular dendritic cell tumor: Review of the entity. Semin Diagn Pathol. 1998. 15: 144-54
12. Sharpe M, Easthope SE, Keating GM, Lamb HM. Polyethylene glycol-liposomal doxorubicin: A review of its use in the management of solid and haematological malignancies and AIDS-related Kaposi's sarcoma. Drugs. 2002. 62: 2089-126
13. Sun X, Chang KC, Abruzzo LV, Lai R, Younes A, Jones D. Epidermal growth factor receptor expression in follicular dendritic cells: A shared feature of follicular dendritic cell sarcoma and Castleman's disease. Hum Pathol. 2003. 34: 835-40
14. Toyoda K, Taniguchi J, Kikawa K, Uike N, Haraoka S, Ooshima K. Follicular dendritic cell sarcoma: Ultrastructural and immunohistochemical studies. Intern Med. 2000. 39: 950-5
15. Wakai S, Yamakawa K, Manaka S, Takakura K. Spontaneous intracranial hemorrhage caused by brain tumor: Its incidence and clinical significance. Neurosurgery. 1982. 10: 437-44
16. Wu A, Pullarkat S. Follicular Dendritic Cell Sarcoma. Arch Pathol Lab Med. 2016. 140: 186-90