- Professor of Clinical Neurosurgery, School of Medicine, State University of N.Y. at Stony Brook, Chief of Neurosurgical Spine and Education, Winthrop NeuroScience, NYU Winthrop Hospital, Mineola, New York – 11501, USA
Correspondence Address:
Nancy E. Epstein
Professor of Clinical Neurosurgery, School of Medicine, State University of N.Y. at Stony Brook, Chief of Neurosurgical Spine and Education, Winthrop NeuroScience, NYU Winthrop Hospital, Mineola, New York – 11501, USA
DOI:10.4103/sni.sni_450_17
Copyright: © 2018 Surgical Neurology International This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Nancy E. Epstein. High cord signals on magnetic resonance and other factors predict poor outcomes of cervical spine surgery: A review. 16-Jan-2018;9:13
How to cite this URL: Nancy E. Epstein. High cord signals on magnetic resonance and other factors predict poor outcomes of cervical spine surgery: A review. 16-Jan-2018;9:13. Available from: http://surgicalneurologyint.com/surgicalint-articles/high-cord-signals-on-magnetic-resonance-and-other-factors-predict-poor-outcomes-of-cervical-spine-surgery-a-review/
Abstract
Background:High cord signals (HCS) on preoperative/postoperative T1, T1 gadolinium-diethylenetriaminepentaacetic acid (Gd-DTPA), and T2 magnetic resonance (MR) studies, postoperative failure of HCS to regress and/or cord re-expansion, and a triangular cord configuration are poor prognostic factors for surgical patients with cervical spondylotic myelopathy (CSM).
Methods:Here, we reviewed the negative prognostic import of high Grades/Types and more extensive locations of preoperative/postoperative HCS on T1, T1 Gd-DTPA, and T2 MR studies in surgical patients with CSM. Additional predictors of poor operative outcomes included postoperative failure of HCS to regress, cord re-expansion at the site of a HCS, and the triangular vs. teardrop or boomerang cord configuration. The Types/Grades of HCS on MR follow:Type/Grade 0 – no/absent signal changes; Type/Grade 1 – mild/light/fuzzy/obscure/low cord signal (LCS) changes; Type/Grade 2 – sharp/intense/well-defined HCS; and Type/Grade 3 – mixed/HCS. The definitions of location/extent of LCS/HCS were: focal (1 level), multifocal (with skip areas), and multisegmental (continuous over >1 segment), while cord configuration was categorized as triangular, teardrop, or boomerang.
Results:On MR studies, preoperative/postoperative Types/Grades 0–1 changes correlated with better prognoses (e.g., improved Japanese Orthopedic Association (JOA) scores or Nurick Grades), while Types/Grades 2–3 correlated with poorer outcomes. Multiple poor prognostic indicators also included; failure of postoperative HCS on MR to regress (particularly if multisegmental), postoperative cord re-expansion at the site of a prior HCS, and triangular cord configuration.
Conclusions:Grade/Types 2–3 HCS on T1, T1 Gd-DTPA, and T2-weighted MR images on preoperative/postoperative MR studies, failure of HCS to regress (multisegmental), cord re-expansion at the site of a prior HCS, and a triangular cord configuration (atrophy) all contributed to poorer outcomes for CSM surgery.
Keywords: Cervical surgery, cord configuration, hyperintense/high cord signal (HCS), ossification of the posterior longitudinal ligament, magnetic resonance, prognostic indicators, spondylotic myelopathy
INTRODUCTION
Here, we reviewed the prognostic import of preoperative/postoperative high cord signals (HCS) on magnetic resonance images [MR: T1, T1 gadolinium-diethylenetriaminepentaacetic acid (Gd-DTPA), and T1] for patients with cervical spondylotic myelopathy. Outcomes were correlated with patients undergoing anteiror cervical diskectomy/fusion (ACDF), anterior corpectomy/fusion (ACF), laminectomy with/without posterior fusion (LAM), and laminoplasty (LOP) [Tables
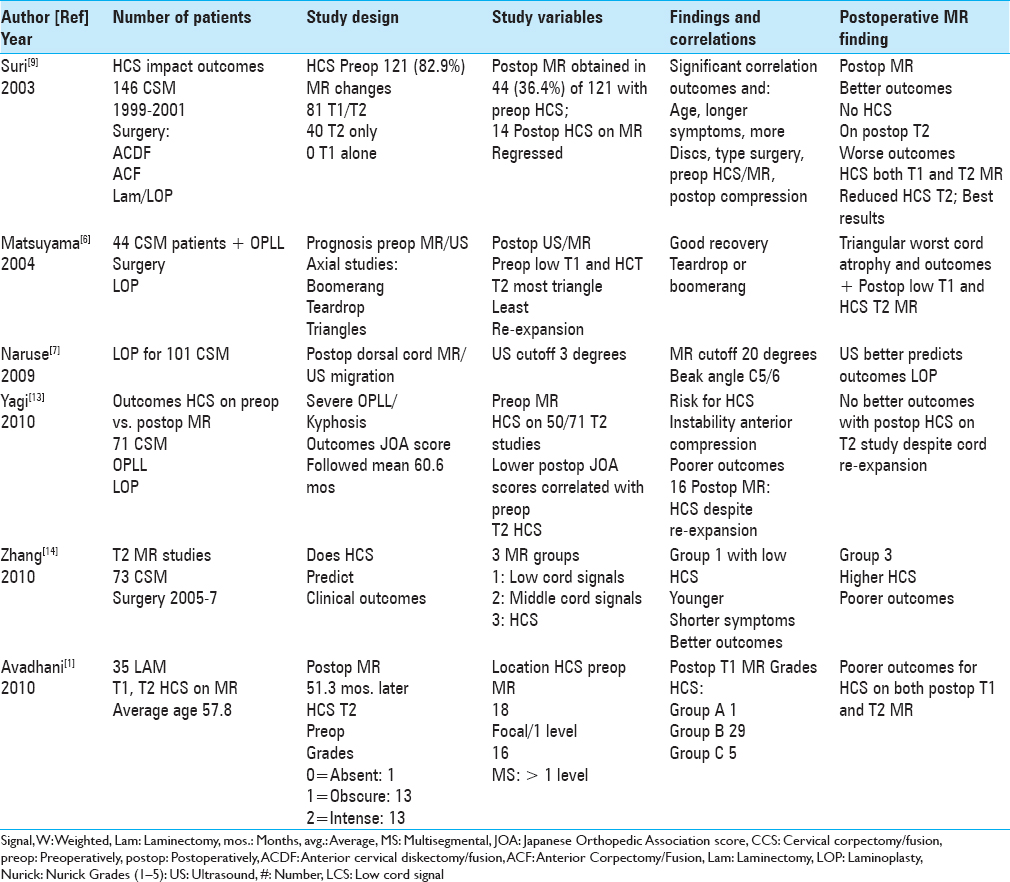
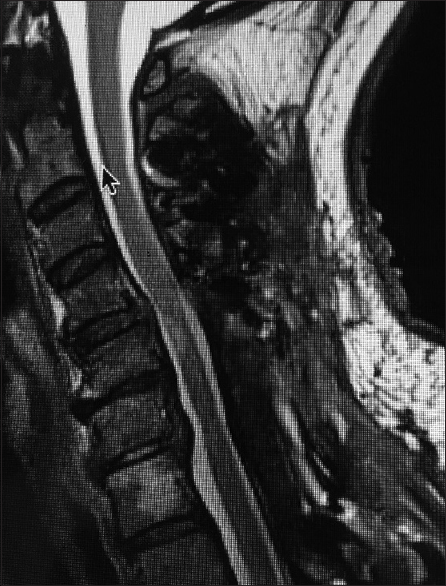
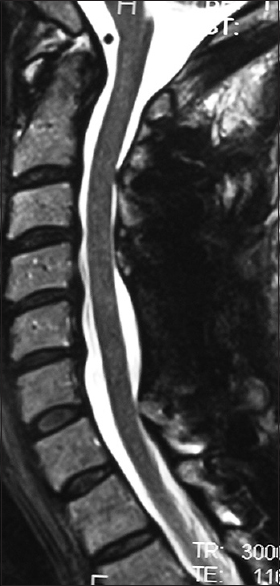
Figure 1
This midline sagittal T2W MR study documented marked cord compression attributed to severe anterior (C4-C6, C6-C7) OPLL, and marked dorsolateral cord compression (C4-C7; ossification of the yellow ligament with laminar shingling). Not the compression was so severe opposite the C5 body that a HCS could not be readily seen on the preoperative T2 MR study
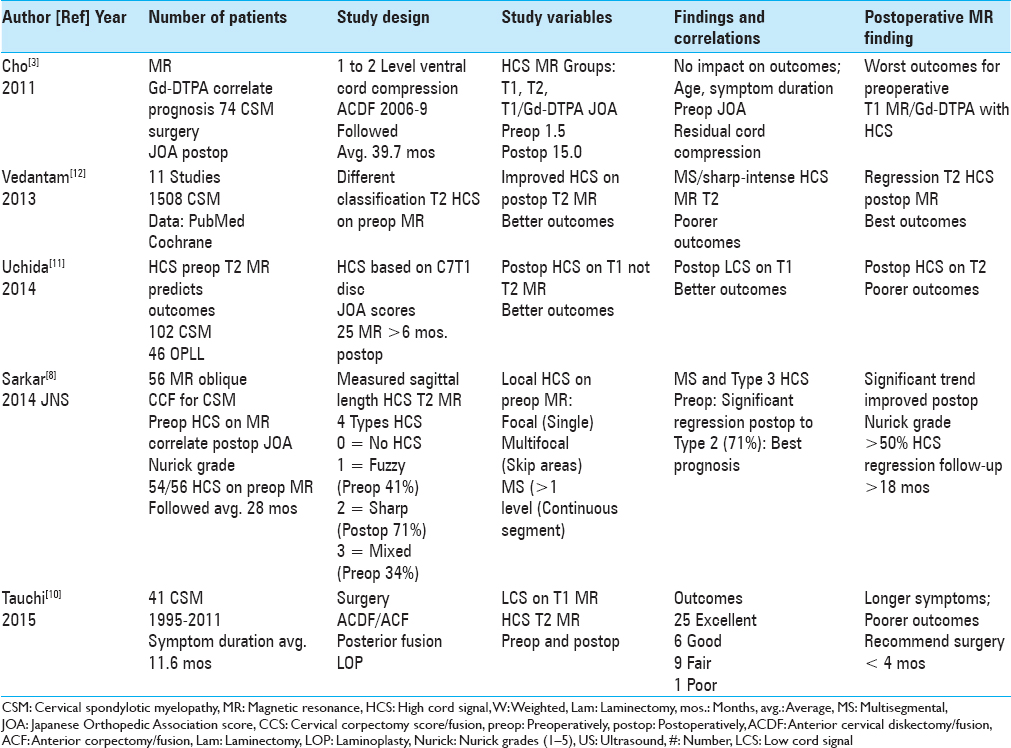
Figure 2
The patient in
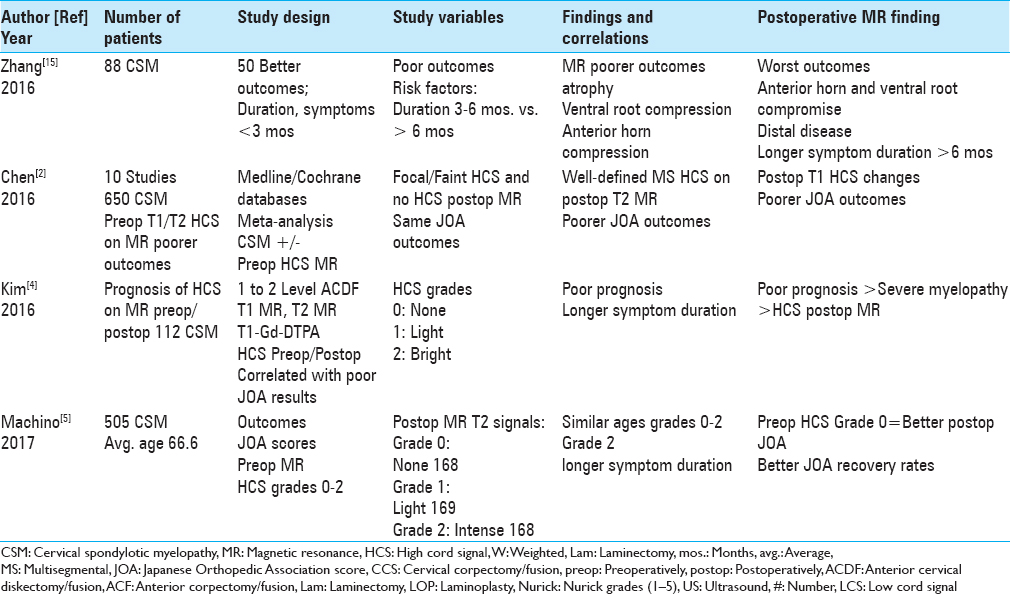
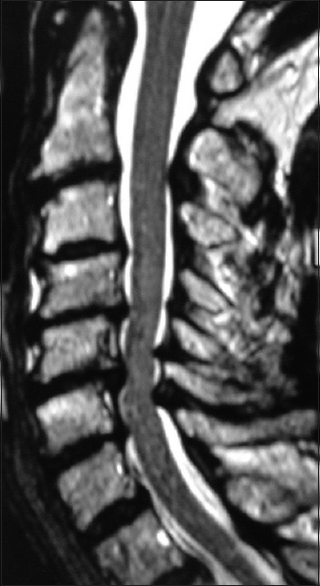
Figure 3
This preoperative midline sagittal T2 MR without a HCS showed moderate anterior osteophytic ridging and maximal dorsolateral cord compression (ossification of the yellow ligament/laminar shingling) from C4-C7 with an excellent cervical lordosis. Here, following a cervical laminectomy of C5-C7, undercutting of C4/T1, and posterior C2-C2 fusion, the patient was fully neurologically intact (Nurick Grade IV to 0)
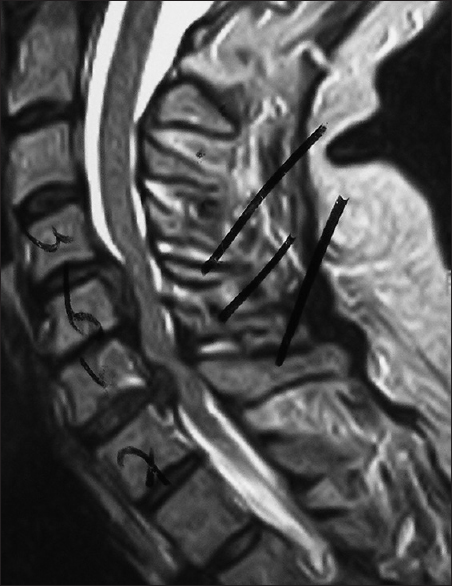
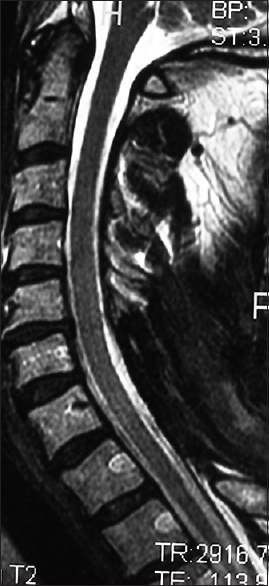
Figure 4
The 6-week postoperative midline sagittal T2 MR in another patient documented adequate cord decompression following a C5-C7 laminectomy, undercutting C4/T1, and posterior C2/T2 fusion. Note, this patient also had no preoperative HCS on the T2 study that correlated with his full postoperative neurological recovery
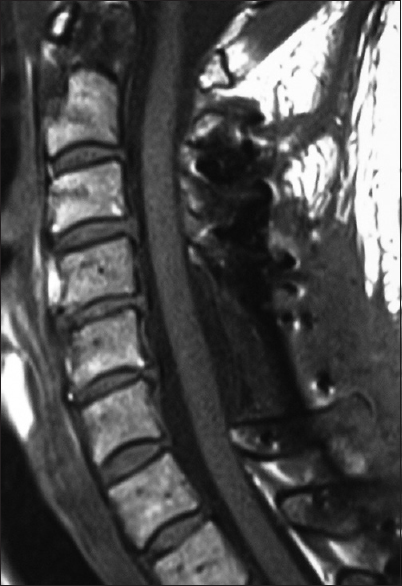
Figure 5
This midline sagittal postoperative 6-week T1 MR documented excellent cord decompression in another patient following a C5-C7 laminectomy, undercutting of C4/T1, with posterior C2/T2 fusion. Note this patient showed neither LCS nor HCS on the preoperative or postoperative T1 or T2 MR studies that nicely correlated with his completely intact neurological status
Figure 6
For the same patient in
Types/grades of HCS on preoperative/postoperative MR studies
Several studies proposed different Types/Grades of HCS identified on preoperative/postoperative T1, T1-DTPA, and T2 MR studies [Tables
Location/extent of HCS and dorsal cord migration on preoperative/postoperative MR studies in CSM patients
Several series also correlated the location/extent of HCS on T2-weighted (T2W) sagittal MR studies with prognoses for CSM surgery [Tables
Prognostic import of axial MR cord shape on outcomes for CSM surgery
Axial MR images documenting different cord configurations (e.g. boomerang, teardrop, or triangular) reflected differing degrees/severity of cord atrophy that also impacted outcomes of CSM surgery [Tables
Better outcomes with Types/Grades 0–1 and worse outcomes with Types/Grades 2–3 HCS on T1/T2 preoperative/postoperative MR studies
On preoperative/postoperative T1/T2 MR studies, Types/Grades 0–1 (no/LCS) correlated with better neurological outcomes, while Types/Grades 2–3 (severe/HCS) correlated with poorer outcomes [Tables
Poorer prognoses for HCS on MR, triangular cord configuration, and cord re-expansion
In several studies, poorer prognoses correlated with preoperative HCS on MR (T1, T1-Gd-DTPA, and T2), preoperative and persistent postoperative triangular cord configuration (reflecting underlying cord atrophy), and cord re-expansion at the site of a prior HCS [Tables
Early surgery recommended for CSM based on MR findings
Tauchi et al. (2015) recommended that CSM patients with preoperative MR studies already showing LCS on T1 and HCS on T2 MR studies, along with extensive/severe stenosis, cord compression, and kyphosis, should be considered for early surgery (within <4 months; whether ACDF, ACF, LOP, LAM) [
CONCLUSION
Here we reviewed multiple MR-based (MR: T1, T2, T1 enahnced studies) prognostic factors for CSM patients undergoing spinal surgery [Tables
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Avadhani A, Rajasekaran S, Shetty AP. Comparison of prognostic value of different MRI classifications of signal intensity change in cervical spondylotic myelopathy. Spine J. 2010. 10: 475-85
2. Chen H, Pan J, Nisar M, Zeng HB, Dai LF, Lou C. The value of preoperative magnetic resonance imaging in predicting postoperative recovery in patients with cervical spondylosis myelopathy: A meta-analysis. Clinics (Sao Paulo). 2016. 71: 179-84
3. Cho YE, Shin JJ, Kim KS, Chin DK, Kuh SU, Lee JH. The relevance of intramedullary high signal intensity and gadolinium (Gd-DTPA) enhancement to the clinical outcome in cervical compressive myelopathy. Eur Spine J. 2011. 20: 2267-74
4. Kim TH, Ha Y, Shin JJ, Cho YE, Lee JH, Cho WH. Signal intensity ratio on magnetic resonance imaging as a prognostic factor in patients with cervical compressive myelopathy. Medicine (Baltimore). 2016. 95: e4649-
5. Machino M, Imagama S, Ando K, Kobayashi K, Ito K, Tsushima M. The Image Diagnostic Classification of MR T2 Increased Signal Intensity in Cervical Spondylotic Myelopathy: Clinical Evaluation Using Quantitative and Objective Assessment. Spine (Phila Pa 1976). 2017. p.
6. Matsuyama Y, Kawakami N, Yanase M, Yoshihara H, Ishiguro N, Kameyama T. Cervical myelopathy due to OPLL: Clinical evaluation by MRI and intraoperative spinal sonography. J Spinal Disord Tech. 2004. 17: 401-4
7. Naruse T, Yanase M, Takahashi H, Horie Y, Ito M, Imaizumi T. Prediction of clinical results of laminoplasty for cervical myelopathy focusing on spinal cord motion in intraoperative ultrasonography and postoperative magnetic resonance imaging. Spine (Phila Pa 1976). 2009. 34: 2634-41
8. Sarkar S, Turel MK, Jacob KS, Chacko AG. The evolution of T2-weighted intramedullary signal changes following ventral decompressive surgery for cervical spondylotic myelopathy: Clinical article. J Neurosurg Spine. 2014. 21: 538-46
9. Suri A, Chabbra RP, Mehta VS, Gaikwad S, Pandey RM. Effect of intramedullary signal changes on the surgical outcome of patients with cervical spondylotic myelopathy. Spine J. 2003. 3: 33-45
10. Tauchi R, Imagama S, Inoh H, Yukawa Y, Kanemura T, Sato K. Appropriate timing of surgical intervention for the proximal type of cervical spondylotic amyotrophy. Eur J Orthop Surg Traumatol. 2015. 25: S107-13
11. Uchida K, Nakajima H, Takeura N, Yayama T, Guerrero AR, Yoshida A. Prognostic value of changes in spinal cord signal intensity on magnetic resonance imaging in patients with cervical compressive myelopathy. Spine J. 2014. 14: 1601-10
12. Vedantam A, Rajshekhar V. Does the type of T2-weighted hyperintensity influence surgical outcome in patients with cervical spondylotic myelopathy? A review. Eur Spine J. 2013. 22: 96-106
13. Yagi M, Ninomiya K, Kihara M, Horiuchi Y. Long-term surgical outcome and risk factors in patients with cervical myelopathy and a change in signal intensity of intramedullary spinal cord on Magnetic Resonance imaging. J Neurosurg Spine. 2010. 12: 59-65
14. Zhang YZ, Shen Y, Wang LF, Ding WY, Xu JX, He . Magnetic resonance T2 image signal intensity ratio and clinical manifestation predict prognosis after surgical intervention for cervical spondylotic myelopathy. J Spine (Phila Pa 1976). 2010. 35: E396-9
15. Zhang J, Cui C, Liu Z, Tong T, Niu R, Shen Y. Predisposing factors for poor outcome of surgery for cervical spondylotic amyotrophy: A multivariate analysis. Sci Rep. 2016. 6: 39512-