- Department of Neurosurgery, University of Cincinnati College of Medicine, and Mayfield Clinic, Cincinnati, Ohio, USA
Correspondence Address:
Robert J. Bohinski
Department of Neurosurgery, University of Cincinnati College of Medicine, and Mayfield Clinic, Cincinnati, Ohio, USA
DOI:10.4103/sni.sni_287_17
Copyright: © 2018 Surgical Neurology International This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.How to cite this article: Justin L. Gibson, Shawn M. Vuong, Robert J. Bohinski. Management of autonomic dysreflexia associated with Charcot spinal arthropathy in a patient with complete spinal cord injury: Case report and review of the literature. 29-May-2018;9:113
How to cite this URL: Justin L. Gibson, Shawn M. Vuong, Robert J. Bohinski. Management of autonomic dysreflexia associated with Charcot spinal arthropathy in a patient with complete spinal cord injury: Case report and review of the literature. 29-May-2018;9:113. Available from: http://surgicalneurologyint.com/surgicalint-articles/management-of-autonomic-dysreflexia-associated-with-charcot-spinal-arthropathy-in-a-patient-with-complete-spinal-cord-injury-case-report-and-review-of-the-literature/
Abstract
Background:Charcot spinal arthropathy (CSA) clearly represents a challenge in long-term spinal cord injury patients, one that can have extremely uncomfortable and potentially lethal outcomes if not managed properly.
Case Description:A 66-year-old man with a history of complete C7 quadriplegia presented with new-onset autonomic dysreflexia that resulted from Charcot spinal arthropathy (CSA). Pathologic instability, in the atypical site of the mid-thoracic spine, spanning from the T8–T9 vertebral levels was appreciated on physical exam as an audible, palpable, and visible dynamic kyphosis; kyphosis was later confirmed on neuroimaging. Based on the CSA severity and sequelae, the patient underwent bilateral decompression laminectomy with lateral extracavitary arthrodesis and posterior instrumentation. Symptoms dramatically improved and at 1-year follow-up, dynamic thoracic kyphosis and most symptoms of autonomic dysreflexia had resolved.
Conclusions:Based on our case and published reports, vigilant imaging and thorough physical examination in long-standing spinal cord injury could help early diagnosis and treatment of CSA, theoretically preventing development of cord atrophy and subsequent long-term sequelae. Surgical correction rather than bracing may be recommended in patients who have complete injury at or above T6 in patients with symptoms of autonomic dysreflexia associated with CSA confirmed on neuroimaging.
Keywords: Charcot spine, complications, long-term spinal cord injury, neuropathic spinal arthropathy, spinal neuroarthropathy
INTRODUCTION
Charcot spinal arthropathy (CSA), also called Charcot spine, spinal neuroarthropathy, or neuropathic spinal arthropathy, is rare mechanical destructive process that affects the intervertebral disc and adjacent vertebral bodies in patients who have loss of joint protective mechanisms.[
CSA should be included in the differential diagnosis of patients with SCI who develop autonomic dysreflexia symptoms with transfers or torso movement.[
Our case highlights a new-onset autonomic dysreflexia that resulted from CSA in a patient with a long history of complete C7 quadriplegia. Following the course from initial presentation to 1-year postoperative examination, we discuss recommendations for treatment based on our case and review of the literature. Vigilant care is needed for patients living with long-standing SCIs, where one can face extremely uncomfortable or potentially lethal outcomes if not managed properly.
CASE REPORT
History
Our patient initially sustained a C5/6 fracture dislocation while playing rugby in 1974 that resulted in complete C7 tetraplegia (as defined by American Spinal Injury Association as ASIA A). Decades later, he came to our clinic with a 3-month history of gradual onset blood pressure fluctuations (up to 240/135) associated with change in position. Concerning to our 66-year-old patient were episodes of other symptoms, including severe headaches, blurry vision, and hyperhidrosis that affected his left arm and face. Neither the blood pressure lability nor symptoms improved with medications (i.e. clonidine, hydralazine, chlorothiazide) or other interventions (i.e. excision of hemorrhoids, colostomy, abdominal exploration with colon resection). Bladder distention, issues with his straight catheterization regimen, anal fissure, and skin ulcers were also all ruled out as potential causes of the autonomic dysreflexia.
Examination
Physical examination noted no voluntary function below the C7 level, good biceps and deltoid power, normal reflexes for C6 and above, and complete sensory level beginning a few inches above the nipple line. An audible, palpable, and visible dynamic kyphosis was seen in the thoracic spine. CT-guided joint aspiration, along with negative findings for gram stain, bacterial culture, acid-fast bacilli testing, and fungal culture, proved negative for indications of discitis or osteomyelitis but were consistent with CSA. In addition, laboratory analysis of blood and urine revealed no signs of sepsis or urinary tract infection.
Imaging
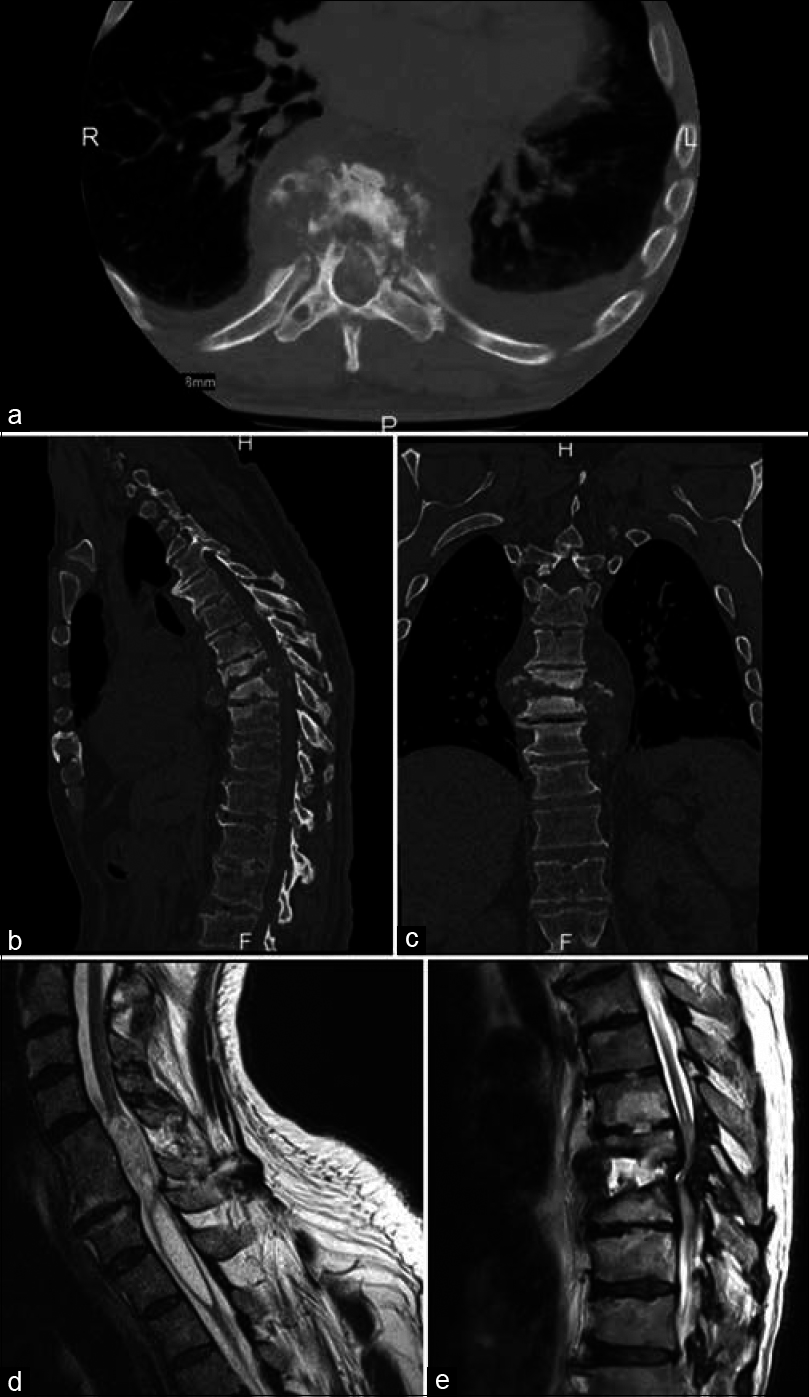
Plain X-ray films showed evidence of mild spondylosis and well-healed intervertebral body fusion of C5-6; mild lumbar spondylosis; and degenerative changes with anterior osteophytes of multiple thoracic levels with no major deformities, spondylolisthesis, or acute compression fractures. CT showed no evidence of an acute or chronic pathologic process in the brain and marked fluid collection was seen in the T8–T9 interspace with destruction of the intervertebral disc space and degradation of the vertebral bodies [
Figure 1
Preoperative imaging in patient with new-onset autonomic dysreflexia and long history of complete C7 quadriplegia. CT imaging in axial (a), sagittal (b), and coronal (c) views showing sclerosis, joint erosion and effusion, paraspinal osseous fluid collection, T8–9 vertebral body destruction with air spaces, and intervertebral disc destruction at the T8–9 interspace. T2-weighted noncontrast MRIs in sagittal view (d and e) shows debris and disorganized fluid within the disc space of T8–9, significant vertebral body erosion of T8–9, complete loss of intervertebral disc integrity at the T8–9 interspace, inflammation and erosion of posterior elements, and proximal syrinx formation from C4–5 to mid-body T2. formation from C4–5 to mid-body T2
Treatment
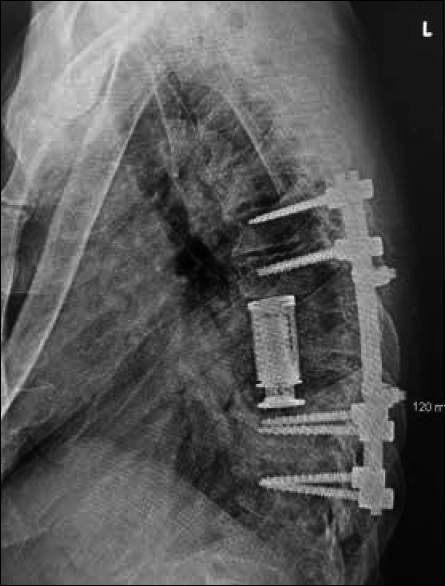
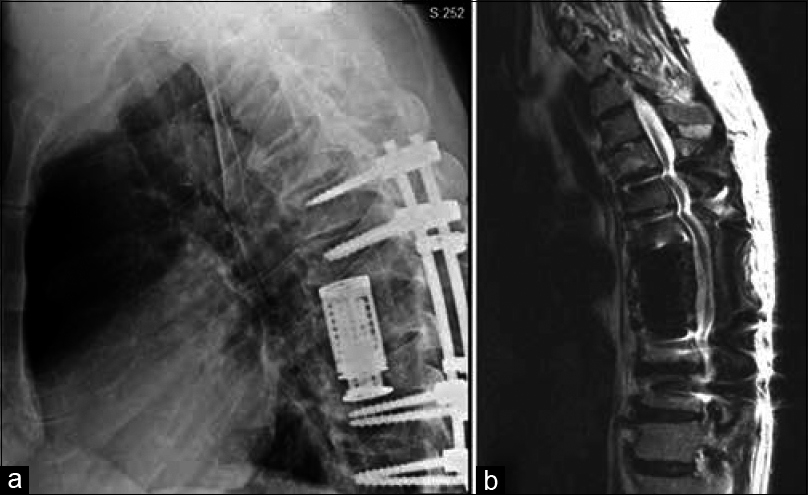
We believed that the cause of the syrinx was secondary to the caudal compression that occurred in the thoracic cord because of the Charcot joint. Given this assessment and the CSA severity and sequelae, the patient underwent surgical decompression of the spinal cord with instrumented arthrodesis. With exposure of the posterior elements from T6 through T11, a hypertrophic reactive mass was identified at the articulation of T8–T9 with benign characteristics on visual inspection. A bilateral decompressive laminectomy of T8 and T9 was then performed, along with discectomies of T7–T8, T8–T9, and T9–T10. The epidural mass associated with the Charcot joint was excised, and both T8 and T9 nerve roots were ligated. Pedicle screws were placed into the T6, T7, T10, and T11 vertebrae, then rods connected and reduced. After a complete lateral extracavitary vertebrectomy of T8 and T9, a titanium vertebral body replacement cage placed into the interspace helped to restore anterior column support and achieve interbody arthrodesis from T7 to T10. Finally, a posterolateral arthrodesis was performed from T6 to T10 using a combination of allograft and autograft bone. Biopsy of the soft tissue and bone at T8–T9 sent for pathologic evaluation was consistent with histological characteristics of CSA.
Postoperative course
The procedure was well tolerated, the postoperative course uncomplicated, and adequate decompression and proper location of all instrumentation confirmed on postoperative imaging [
DISCUSSION
Our patient's urgent symptoms of autonomic dysreflexia were associated with a relatively rare CSA owing to the effects of a long-standing spinal cord injury. Most reported cases of CSA have been found at the thoracolumbar and lumbosacral junctions[
Charcot joint, a disease progression
After its initial description by Charcot, the term Charcot joint emerged based on the mid-19th century work that demonstrated an association with tabes dorsalis.[
The time between onset of neurological impairment and diagnosis of CSA averages 17.3 years.[
Charcot spinal arthropathy, considerations in long-term care of spinal cord injury
Our CSA patient's urgent symptoms of autonomic dysreflexia highlight one complexity for SCI patients aiming to live actively long-term. Although the most common cause of autonomic dysreflexia is genitourinary affecting the bladder, large bowel, rectum, or anal canal, other causes include infection, muscle spasms, trauma, and syringomyelia.[
Typical, though nonspecific, imaging characteristics of CSA include the “six Ds:” distension (soft-tissue mass), density (sclerosis with preserved bone density), debris (osseous fragments), disorganization (articular contour distortion with intervertebral joint abnormalities), dislocation (spondylolisthesis), and destruction (of endplates and facets).[
CONCLUSIONS
Vigilant, routine imaging, and thorough physical examination in patients with long-standing SCI can aid early diagnosis and treatment, thus theoretically preventing the development of cord atrophy and subsequent long-term sequelae. We believe that symptomatic CSA is best managed with surgical stabilization of the affected joint. In our patient and several other reports, the cervical syrinx resolved after cord decompression, which may contribute to the observed positive outcomes. In asymptomatic CSA, either conservative or surgical treatments can be appropriate, largely based on patient preferences and comorbidities. CSA clearly represents a challenge in long-term spinal cord injury patients, one that can have extremely uncomfortable and potentially lethal outcomes if not managed properly.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient has given her consent for her images and other clinical information to be reported in the journal. The patient understands that name and initial will not be published and due efforts will be made to conceal identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Arnold JM, Feng QP, Delaney GA, Teasell RW. Autonomic dysreflexia in tetraplegic patients: Evidence for alpha-adrenoceptor hyper-responsiveness. Clin Auton Res. 1995. 5: 267-70
2. Baker N, Green A, Krishnan S, Rayman G. Microvascular and C-fiber function in diabetic charcot neuroarthropathy and diabetic peripheral neuropathy. Diabetes Care. 2007. 30: 3077-9
3. Barrey C, Massourides H, Cotton F, Perrin G, Rode G. Charcot spine: Two new case reports and a systematic review of 109 clinical cases from the literature. Ann Phys Rehabil Med. 2010. 53: 200-20
4. Beard JP, Wade WH, Barber DB. Sacral insufficiency stress fracture as etiology of positional autonomic dysreflexia: Case report. Paraplegia. 1996. 34: 173-5
5. Bloch R, Bloch RF, Basbaum M.editors. Autonomic dysfunction in management of spinal cord. Management of Spinal Cord Injuries. Baltimore, MD: Williams and Wilkins; 1986. p. 149-63
6. Brown R, Burton A, Macefield VG. Input-output relationships of a somatosympathetic reflex in human spinal injury. Clin Auton Res. 2009. 19: 213-20
7. Charcot J. Sur quelques arthropathies qui paraissent dépendre d'une lésion du cerveau ou de la moelle épiniére. Arch Physiol Norm Pathol. 1868. 1: 161-78
8. Crim JR, Bassett LW, Gold RH, Mirra JM, Mikulics M, Dawson EG. Spinal neuroarthropathy after traumatic paraplegia. Am J Neuroradiol. 1988. 9: 359-62
9. Curt A, Nitsche B, Rodic B, Schurch B, Dietz V. Assessment of autonomic dysreflexia in patients with spinal cord injury. J Neurol Neurosurg Psych. 1997. 62: 473-7
10. Devlin VJ, Ogilvie JW, Transfeldt EE, Boachie-Adjei O, Bradford DS. Surgical treatment of neuropathic spinal arthropathy. J Spinal Disord. 1991. 4: 319-28
11. Glennon TP, Madewell JE, Donovan WH, Bontke CF, Spjut HJ. Neuropathic spinal arthropathy after spinal cord injury. A report of three cases. Spine (Phila Pa 1976). 1992. 17: 964-71
12. Guttman L, Whitteridge D. Effects of bladder distension on autonomic mechanism after spinal cord injuries. Brain. 1947. 70: 361-404
13. Karlsson AK. Autonomic dysreflexia. Spinal Cord. 1999. 37: 383-91
14. Key J. Clinical observations of tabetic arthropathies (Charcot joints). Am J Syph. 1932. 16: 429-
15. Kronig G. Spondylolisthese bei einem Tabiker. Zeit Klin Med. 1884. 7: 5-6
16. Lacout A, Lebreton C, Mompoint D, Mokhtari S, Vallee CA, Carlier RY. CT and MRI of spinal neuroarthropathy. AJR Am J Roentgenol. 2009. 193: W505-514
17. Larson SA, Burns PR. The pathogenesis of Charcot neuroarthropathy: Current concepts. Diabet Foot Ankle. 2012. 3:
18. Ledbetter LN, Salzman KL, Sanders RK, Shah LM. Spinal neuroarthropathy: Pathophysiology, clinical and imaging features, and differential diagnosis. Radiographics. 2016. 36: 783-99
19. Mascarenhas JV, Jude EB. Pathogenesis and medical management of diabetic Charcot neuroarthropathy. Med Clin North Am. 2013. 97: 857-72
20. Mathias C, Frankel H, Mathias C, Bannister R.editors. Autonomic disturbances in spinal cord lesions. Autonomic failure: A textbook of clinical disorders of the autonomic nervous system. Oxford: Oxford University Press; 1999. p. 494-513
21. Mathias C, Frankel H, Cole J, Illis L.editors. Management of cardiovascular abnormalities caused by autonomic dysfunction in spinal cord injury. Spinal Cord Dysfunction: Intervention and Treatment. Oxford: Oxford University Press; 1992. p. 101-20
22. McNeel DP, Ehni G. Charcot joint of the lumbar spine. J Neurosurg. 1969. 30: 55-61
23. Milligan J, Lee J, McMillan C, Klassen H. Autonomic dysreflexia: Recognizing a common serious condition in patients with spinal cord injury. Can Fam Physician. 2012. 58: 831-5
24. Mohit AA, Mirza S, James J, Goodkin R. Charcot arthropathy in relation to autonomic dysreflexia in spinal cord injury: Case report and review of the literature. J Neurosurg Spine. 2005. 2: 476-80
25. Montgomery TJ, McGuire RA. Traumatic neuropathic arthropathy of the spine. Orthop Rev. 1993. 22: 1153-7
26. Morita M, Miyauchi A, Okuda S, Oda T, Yamamoto T, Iwasaki M. Charcot spinal disease after spinal cord injury. J Neurosurg Spine. 2008. 9: 419-26
27. Morita M, Iwasaki M, Okuda S, Oda T, Miyauchi A. Autonomic dysreflexia associated with Charcot spine following spinal cord injury: A case report and literature review. Eur Spine J. 2010. 19: S179-82
28. Park YH, Taylor JA, Szollar SM, Resnick D. Imaging findings in spinal neuroarthropathy. Spine (Phila Pa 1976). 1994. 19: 1499-504
29. Phillips S, Williams AL, Peters JR. Neuropathic arthropathy of the spine in diabetes. Diabetes Care. 1995. 18: 867-69
30. Race MC, Keppler JP, Grant AE. Diabetic Charcot spine as cauda equina syndrome: An unusual presentation. Arch Phys Med Rehabil. 1985. 66: 463-5
31. Selmi F, Frankel HL, Kumaraguru AP, Apostopoulos V. Charcot joint of the spine, a cause of autonomic dysreflexia in spinal cord injured patients. Spinal Cord. 2002. 40: 481-3
32. Slabaugh PB, Smith TK. Neuropathic spine after spinal cord injury. A case report. J Bone Joint Surg Am. 1978. 60: 1005-6
33. Sobel JW, Bohlman HH, Freehafer AA. Charcot's arthropathy of the spine following spinal cord injury. A report of five cases. J Bone Joint Surg Am. 1985. 67: 771-6
34. Standaert C, Cardenas DD, Anderson P. Charcot spine as a late complication of traumatic spinal cord injury. Arch Phys Med Rehabil. 1997. 78: 221-5
35. Wagner SC, Schweitzer ME, Morrison WB, Przybylski GJ, Parker L. Can imaging findings help differentiate spinal neuropathic arthropathy from disk space infection? Initial experience. Radiology. 2000. 214: 693-9
36. Young MJ, Marshall A, Adams JE, Selby PL, Boulton AJ. Osteopenia, neurological dysfunction, and the development of Charcot neuroarthropathy. Diabetes Care. 1995. 18: 34-8
37. Zucker G, Marder MJ. Charcot spine due to diabetic neuropathy. Am J Med. 1952. 12: 118-24