- Department of Neurosurgery, Clinical Neurosciences Center, University of Utah School of Medicine, Salt Lake City, Utah, USA
Correspondence Address:
M. Yashar S. Kalani
Department of Neurosurgery, Clinical Neurosciences Center, University of Utah School of Medicine, Salt Lake City, Utah, USA
DOI:10.4103/sni.sni_475_16
Copyright: © 2017 Surgical Neurology International This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Nicholas T. Gamboa, Philipp Taussky, Min S. Park, William T. Couldwell, Mark A. Mahan, M. Yashar S. Kalani. Neurovascular patterning cues and implications for central and peripheral neurological disease. 06-Sep-2017;8:208
How to cite this URL: Nicholas T. Gamboa, Philipp Taussky, Min S. Park, William T. Couldwell, Mark A. Mahan, M. Yashar S. Kalani. Neurovascular patterning cues and implications for central and peripheral neurological disease. 06-Sep-2017;8:208. Available from: http://surgicalneurologyint.com/surgicalint-articles/neurovascular-patterning-cues-and-implications-for-central-and-peripheral-neurological-disease/
Abstract
The highly branched nervous and vascular systems run along parallel trajectories throughout the human body. This stereotyped pattern of branching shared by the nervous and vascular systems stems from a common reliance on specific cues critical to both neurogenesis and angiogenesis. Continually emerging evidence supports the notion of later-evolving vascular networks co-opting neural molecular mechanisms to ensure close proximity and adequate delivery of oxygen and nutrients to nervous tissue. As our understanding of these biologic pathways and their phenotypic manifestations continues to advance, identification of where pathways go awry will provide critical insight into central and peripheral nervous system pathology.
Keywords: Angiogenesis, axon guidance, neurogenesis, neurosurgery, vascular endothelial growth factor
INTRODUCTION
The ability to perceive and integrate multiple sensory inputs and produce an appropriate and directed response explains much of the evolutionary success of kingdom Animalia. Neurons began as specialized cells capable of generating electrochemical gradients and propagating electric potentials to neighboring cells. As primitive nervous systems evolved, from simple nerve nets to distinct nerve cords with eventual cephalization, the parallel branching of vascular channels made development of the human central and peripheral nervous systems possible.[
The increasing efficiency and complexity of evolving nervous systems necessitated greater metabolic demands and distributive capacity of the organism. During development, patterning cues generate rostrocaudal and dorsoventral domains that ultimately go on to differentiate into tissues and organs. Given the graded complexity and rapid cycles of proliferation necessary to generate the cell required for specification of tissues and organs, respiring organisms have developed expansive parallel vascular networks (consisting of arteries, veins, and capillaries) capable of delivering oxygen and nutrients and removing waste from nerve tissue [
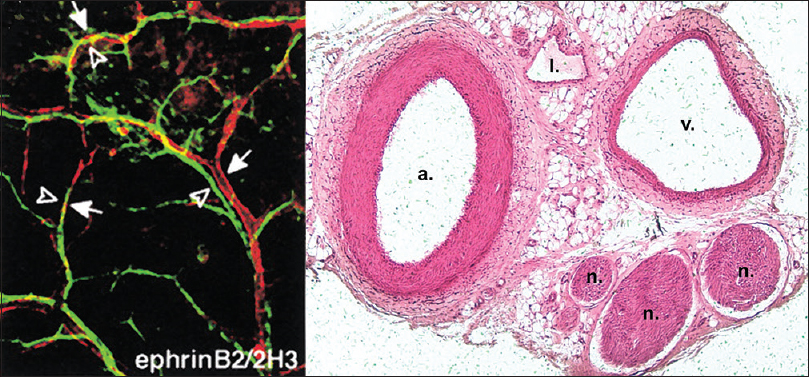
Figure 1
Parallel alignment of developing arteries and nerves. (Left) Whole-mount immunofluorescence confocal microscopy with antibodies to endothelial marker PECAM-1 and neuronal marker Tuj-1. Note the coalignment of main sensory nerves (green) with their arteries (red). Reproduced with permission.[
Evidence continues to emerge demonstrating how neuronal axon growth, branching and arborization, and angiogenesis rely on similar growth factors and receptors for their parallel and seemingly intertwined development. As more complex neuronal circuitry evolved, it seems that the later-evolving vascular networks may have co-opted their molecular mechanisms to ensure close proximity and adequate delivery of oxygen and nutrients to traveling nerves. In this review, we examine the similarities and differences between neurogenesis and angiogenesis, the current evidence regarding their mechanisms, their reliance on one another for normal physiology, and the aberrancies in these processes that precipitate neurosurgical pathology.
NEUROGENESIS
Axonal growth cones
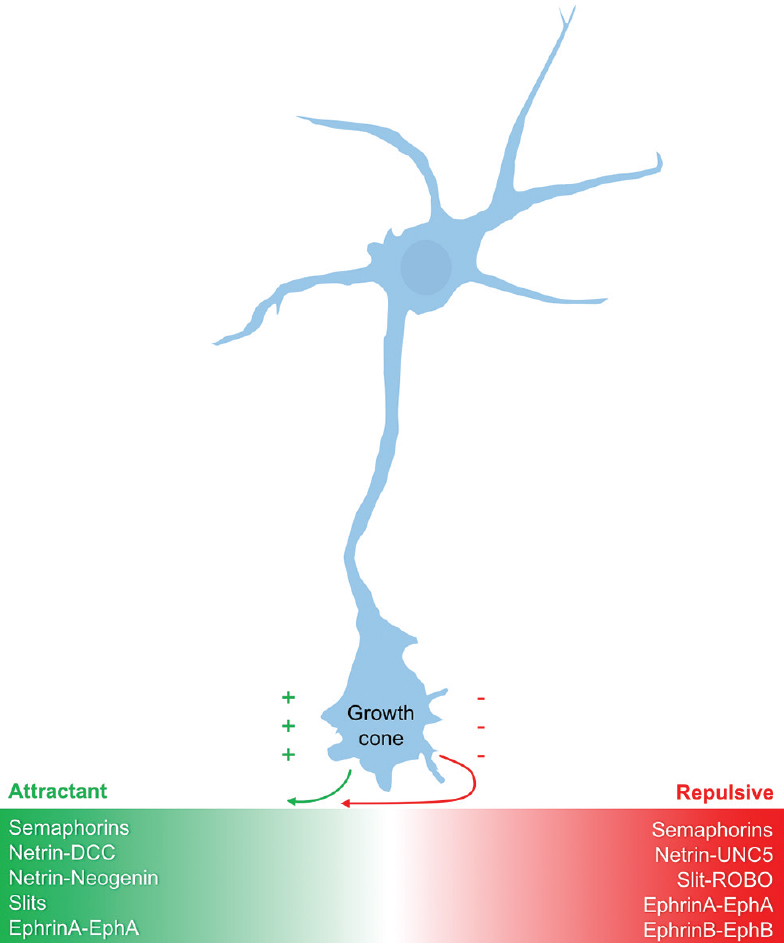
The nervous and vascular systems appear grossly similar, consisting of highly branched networks that parallel one another throughout the human body; however, at a microscopic level, their initial formation appears quite distinct. Neurons begin by thrusting a long axon outward, headed by the sensory neuronal growth cone. The path of this growth cone is dictated largely by attractant and repulsive guidance proteins secreted by individual target cells along with the specific expression pattern of receptors on the growth cone itself.[
Modern genetic and molecular techniques have revealed highly conserved families of guidance molecules involved in axonal guidance. These guidance molecules can either attract or repel the neuronal growth cone, are capable of operating over both short and long distances, and can influence the bundling of axons together into nerve fascicles.[
Semaphorins
Semaphorins are a large, diverse, and phylogenetically conserved family of both secreted and membrane-associated proteins.[
Netrins
Netrins are a small family of evolutionarily conserved proteins that are either secreted (netrin-1, netrin-3, netrin-4) or membrane-bound via glycosylphosphatidylinositol (GPI)-anchoring (netrin-G1, netrin-G2). Netrins were first identified in studies of Caenorhabditis elegans as ventral midline-derived chemoattractants that helped guide axons to the midline through binding to the DCC (deleted in colorectal carcinoma) family of receptors.[
Slits
During development of the embryonic nervous system, commissural axons are initially attracted by cues derived from netrin-DCC interaction. Once axons are at the midline where netrin levels are highest, this attractive signal must be silenced to prevent stalling or recrossing. This silencing is mediated largely by Slit proteins, which, like netrins, are also made by ventral midline cells in the developing embryo.[
Slits are a family of large secreted glycoproteins initially discovered for their repellant effects in Drosophila melanogaster (fruit fly) axons crossing the ventral midline, but they have also shown dual functionality as attractant cues to navigating axons.[
Ephrins
Eph receptor tyrosine kinases (RTKs) and their membrane-bound ligands, the ephrins, act principally as short-range axon guidance molecules and play important roles in the developing nervous system through their effects on axon guidance and synaptogenesis.[
MORPHOGENS
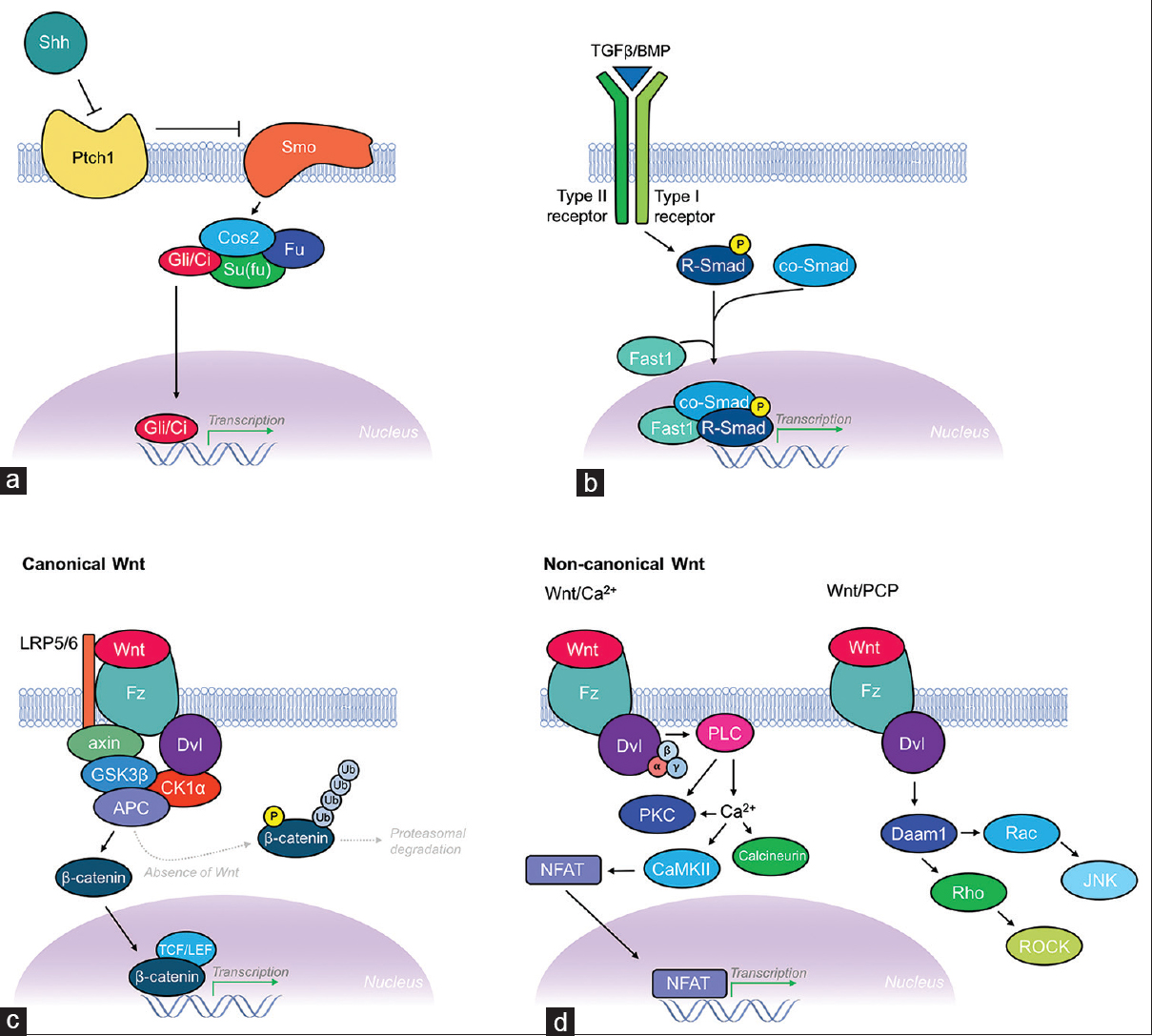
Morphogens are signaling factors that direct cell fate and tissue development in a restricted region of tissue by providing gradient-mediated positional information. Morphogens exert their effects by being produced in a particular region of tissue and then diffusing from this source, thereby establishing gradients. The asymmetry of gradients produced by morphogens allows for production of different cell types across the gradient. This is further complicated by overlapping regions of signaling gradients produced by multiple morphogens. Two factors determine whether a secreted protein can be classified as a morphogen: first, it must act in a concentration-dependent manner on its target cells/tissues; and second, it must exert a direct effect from a distance. A large number of morphogens have been identified to date, although the canonical morphogen families include the hedgehog (Hh), Decapentaplegic (DPP)/transforming growth factor-β (TGF-β)/bone morphogenetic proteins (BMPs), and Wnt signaling pathways [
Figure 3
Schematic illustration of the Shh, TGF-β/BMP, and Wnt morphogenic signaling pathways. (a) Shh signaling pathway. Hhs like Shh are known to activate signaling through binding their receptor Patched (Ptch1; a 12-pass transmembrane protein). This leads to relief of inhibition of Smoothened (Smo; a 7-pass transmembrane protein), which then leads to a downstream intracellular signaling cascade. Smo then associates with the Gli/Ci-containing complex, which includes Costal 2 (Cos2), and the protein kinase Fused (Fu) and Su (fu) (suppressor of fused). Together, this complex acts constitutively to suppress the pathway by activating proteolysis of Gli/Ci, thus acting as a transcriptional repressor. Activation of Hh signaling reverses this regulatory inhibition of Gli/Ci, allowing transcription of Hh target genes. (b) TGF-β/BMP signaling pathway. Members of the DPP/BMP/TGF-β family of morphogens regulate cell fate and proliferation through binding to the extracellular domain of type I and type II TGF-β receptors, causing dimerization and autophosphorylation of the type I receptor's intracellular kinase domain. Targets of the type I receptor are the receptor-regulated Smads (R-Smads), which are subsequently phosphorylated inducing their association with co-Smads before translocating to the nucleus where they combine with other DNA-binding proteins (Fast1) to initiate transcription of TGF-β/BMP target genes. (c) Canonical Wnt signaling pathway. The canonical Wnt signaling pathway (β-catenin dependent) pathway controls gene expression through stabilization of intracellular β-catenin. Binding of Wnt to its receptor Frizzled (Fz; a 7-pass transmembrane protein), with coreceptor LRP-5/6, leads to Dishevelled (Dsh) activation and suppression of GSK3β activity, thus preventing phosphorylation, ubiquitination, and proteasomal degradation of β-catenin. This requires formation of a complex scaffolded by axin and adenomatous polyposis coli (APC) proteins. Increased concentrations of β-catenin transform lymphoid enhancer factor (LEF)/T-cell factor (TCF) from transcriptional repressor to activator thereby leading to transcription of Wnt target genes. (d) Non-canonical signaling pathways. The two non-canonical Wnt signaling pathways include the Wnt/Ca2+ pathway and the Wnt/planar cell polarity (PCP) pathway. The Wnt/Ca2+ pathway also involves binding of Wnt to Fz and subsequent Dsh activation, but instead signals via heterotrimeric G-proteins (α, β, γ subunits) leading to activation of phospholipase C (PLC) and increased intracellular Ca2+ concentrations, while simultaneously activating protein kinase C (PKC). Increased Ca2+ leads to activation of calcineurin and CaMKII. CaMKII induces activation of the transcription factor NFAT, which leads to transcription of Wnt/Ca2+ target genes involved in cell adhesion and migration. The Wnt/PCP pathway also involves Wnt binding Fz leading to recruitment and activation of Dsh, which then forms a complex with Dishevelled-associated activator of morphogenesis 1 (Daam1). Daam1 subsequently activates the G-protein Rho, which leads to activation of Rho-associated kinase (ROCK), a major regulator of the cellular cytoskeleton. Dsh also forms a complex with Rac, which activates JNK and leads to actin polymerization
Hedgehog family
In the early 1980s, the fundamental problem in developmental biology of how a single-celled zygote could give rise to complex, highly organized, segmented organs and tissues was solved through the discovery of mutations in genes controlling anterior–posterior body axis polarization in Drosophila embryogenesis.[
Transforming growth factor-β family
DPP, BMP, and TGF-β are all members of the TGF-β superfamily of morphogens. About the time dorsal neurons are formed at the dorsal midline of the developing embryo, roof plate cells express many of these members of the TGF-β family as they are required for the dorsal specification of developing neurons.[
BMPs are known to guide commissural axons through type I and type II TGF-β receptors. In addition, the individual receptor subunits are thought to play a role in downstream signaling events in axon guidance, thus differing specification of cell fate. BMP7:GDF7 heterodimers that are secreted by the roof plate cells have been shown to repel commissural axons ventrally and are also capable of inducing collapse of commissural axon growth cones.[
Wnt family
Wnts are a large family of 19 highly conserved glycoproteins that have three known signal transduction pathways and can initiate different intracellular signaling cascades determining cell fate, proliferation, migration, and polarity. Wnt signaling pathways can be classified into canonical (β-catenin dependent) and noncanonical (β-catenin independent).[
Wnts have been shown to act as axonal guidance cues for post-midline crossing commissural and corpus collosal axons,[
VASCULAR PATTERNING
Vascular development consists of two disparate yet closely interconnected developmental programs – vasculogenesis and angiogenesis. Vasculogenesis is the development of vascular beds from progenitor cells early in the development, whereas angiogenesis is the sprouting of new vessels from pre-existing vasculature. Each of these processes and the signaling cues regulating them will be discussed further below.
Vasculogenesis
Whereas individual axons can traverse vast distances, as evinced by the sciatic nerve, endothelial cells take a more modest approach. Although they cannot individually travel as far, the assembly and proliferation of endothelial cells allows them to mirror the movements of neuronal axons. Vasculogenesis begins with the differentiation of vascular progenitor cells, termed angioblasts, into endothelial cells that migrate and coalesce to form primitive vascular cords.[
Similar to the glial cells supporting the neuronal circuitry of the cerebrum, the endothelial cells rely heavily on vascular smooth muscle cells and pericytes for their growth, maturation, and vessel stabilization. Soon after differentiating, the endothelial cells begin to secrete platelet-derived growth factor (PDGF) to recruit vascular smooth muscle cells from the surrounding mesenchymal and neural crest-derived embryonic tissue.[
Angiogenesis induction
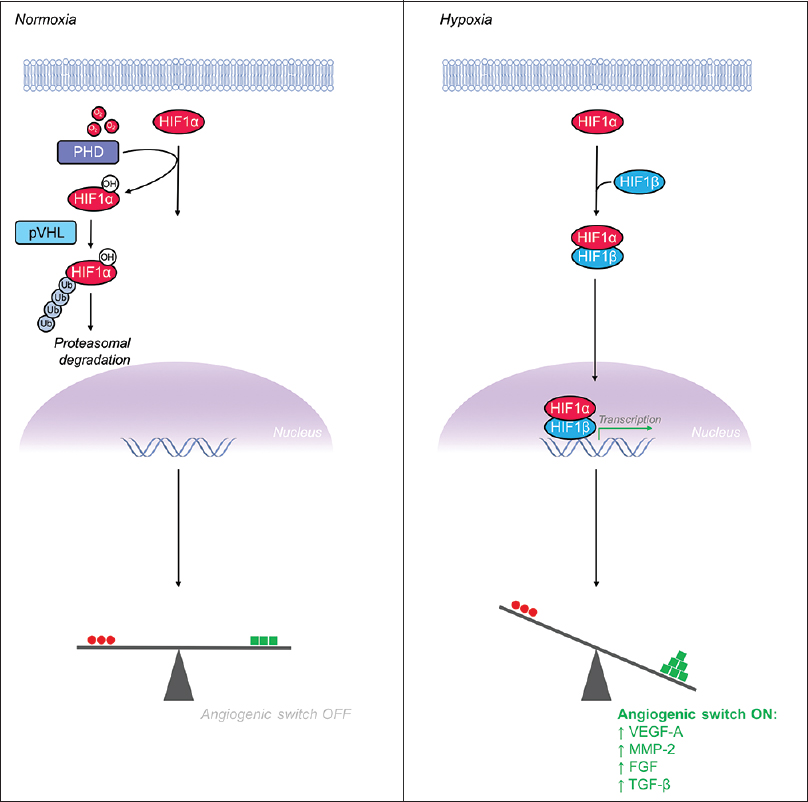
Given the rapidly changing metabolic needs of various tissues throughout the human body, the vascular system has evolved mechanisms to meet the oxygen and nutrient requirements of nearby respiring tissues. Angiogenesis, which is the sprouting of new vessels from pre-existing vasculature, allows nearby blood vessels to sense tissue hypoxia and respond appropriately.[
Figure 4
Hypoxia inducible factor-1α (HIF-1α) is a major transcriptional regulator whose levels increase in hypoxia, leading to flipping the “angiogenic switch” on. (Left) Under normoxic conditions, HIF-1α is hydroxylated by prolyl hydroxylase (PHD) enzymes and the VHL-mediated ubiquitin proteasome pathway rapidly degrades HIF-1α, maintaining low levels of intracellular HIF-1α. (Right) Under hypoxic conditions, the PHD enzymes, which require oxygen as a substrate, are unable to hydroxylate HIF-1α's proline residue thus leading to escape from the degradation pathway and increased levels intracellular HIF-1α. Accumulation of HIF-1α leads to formation of a heterodimer with HIF-1β before translocating to the nucleus to serve as a potent activator of pro-angiogenic gene expression
VEGF-A stimulates endothelial cell proliferation and migration and is critical for both vasculogenesis and angiogenesis. VEGF-A induces angiogenesis through binding to its primary tyrosine kinase receptor VEGFR2 and initiating the RAS/RAF/MEK/ERK signaling cascade.[
Sprouting and tip cell selection
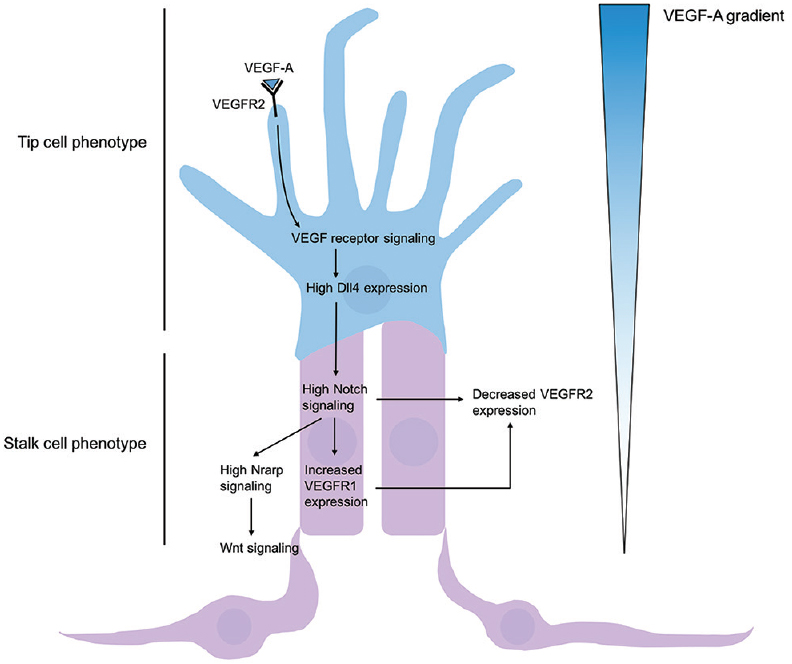
Capillary endothelial cells, much like the neuronal growth cones, are capable of sensing and responding to environmental cues by sprouting and growing towards chemotactic signals. Initially, quiescent endothelial cells specify into tip and stalk cells in a process controlled largely via the Notch pathway.[
Figure 5
Regulation of tip and stalk cell formation. VEGF-A gradient determines tip cell selection, and subsequent Dll4-Notch signaling induces stalk cell phenotype of nearby endothelium. High concentrations of VEGF-A bind and activate VEGFR2, leading to increased expression of membrane-restricted Dll4 in the tip cell. Dll4 acts in a juxtacrine manner with Notch1 receptors, thus promoting Notch signaling of adjacent epithelium and leading to gene expression promoting a stalk cell phenotype. High Notch signaling leads to high Notch-regulated ankyrin repeat protein (Nrarp) and Wnt signaling
ABERRANT SIGNALING IN NEUROVASCULAR PATHOLOGY
The intimate association and codependency of nervous and vascular tissue in the central and peripheral nervous systems is essential for normal development and physiology. Aberrancies in these processes drive much of neurosurgical pathology. Through a better understanding of the mechanisms underlying normal physiology of nervous and vascular tissues, understanding of dysregulation from a genetic and molecular approach can lead to new therapeutics or treatment approaches for neurosurgical patients.
Arteriovenous malformations
Arteriovenous malformations (AVMs) are vascular lesions that are characterized by a tangle of abnormal vessels that directly shunt blood from arterial to venous circulation without an interposed capillary bed. Cerebral AVMs most commonly occur sporadically but can also be associated with genetic disorders such as hereditary hemorrhagic telangiectasia (HHT) (Osler–Weber–Rendu disease), Wyburn–Mason syndrome, or Sturge–Weber syndrome.[
The behavioral heterogeneity of AVMs is thought to stem largely from their altered gene expression.[
In addition to genetic mutations contributing to arteriovenous pathology, the AVM microenvironment itself is thought to contribute to further stimulation of pathologic angiogenesis. Because AVMs act as a pathologic shunt, both ischemia and hypoxia precipitate HIF-1α accumulation, activating the angiogenic switch. Experiments have demonstrated that this hypoxic microenvironment surrounding the AVM can lead to a substantial increase in VEGF (up to 30-fold).[
Glioblastoma
Despite advances in technology, surgical technique, and medical therapies, glioblastoma (GBM; WHO Grade IV astrocytoma) remains a lethal disease with rapid progression and inevitable recurrence after conventional therapy with maximal safe surgical resection and subsequent radiation therapy with concurrent temozolomide. Yet, despite its uniformly aggressive phenotype, a hallmark of this particular disease is its genetic heterogeneity. VEGF, HIF-1α, PDGF, TGF-β, FGF, and epidermal growth factor (EGF) all play critical roles in pathologic angiogenesis, a characteristic feature of GBMs.[
Another important growth factor in GBM progression involves TGF-β, which has been demonstrated to be involved in cellular proliferation, differentiation, and apoptotic resistance of tumor cells.[
Vestibular schwannomas
Vestibular schwannomas (or acoustic neuromas) are benign intracranial tumors of the myelin-forming Schwann cells ensheathing the eighth cranial nerves. Schwannomas have low malignant potential and often occur in the head and neck (25–40%) but can occur elsewhere in the body.[
CONCLUSIONS AND PERSPECTIVES
Nerves and vasculature follow parallel paths with overlapping anatomy, supplying electrical impulses and much-needed oxygen and nutrients throughout the human body, respectively. The gross organizational similarity between the nervous and vascular systems is evinced by a highly stereotyped pattern of branching that mirrors one another as they travel to supply their target tissues throughout the body. In addition, the parallels between these two systems extend to a genetic and molecular level where evidence of their relatedness and interplay between these two systems continues to accumulate. Through a better understanding of the development of neurovascular pathways and the aberrancies precipitating their pathology, new therapeutic targets will likely be identified.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Adams RH, Alitalo K. Molecular regulation of angiogenesis and lymphangiogenesis. Nat Rev Mol Cell Biol. 2007. 8: 464-78
2. Adams RH, Eichmann A. Axon guidance molecules in vascular patterning. Cold Spring Harb Perspect Biol. 2010. 2: a001875-
3. Adams RH, Wilkinson GA, Weiss C, Diella F, Gale NW, Deutsch U. Roles of ephrinB ligands and EphB receptors in cardiovascular development: Demarcation of arterial/venous domains, vascular morphogenesis, and sprouting angiogenesis. Genes Dev. 1999. 13: 295-306
4. Ahmad Z, Brown CM, Patel AK, Ryan AF, Ongkeko R, Doherty JK. Merlin knockdown in human Schwann cells: Clues to vestibular schwannoma tumorigenesis. Otol Neurotol. 2010. 31: 460-6
5. Ajiboye N, Chalouhi N, Starke RM, Zanaty M, Bell R. Cerebral arteriovenous malformations: Evaluation and management. ScientificWorldJournal 2014. 2014. p. 649036-
6. Allen BL, Song JY, Izzi L, Althaus IW, Kang JS, Charron F. Overlapping roles and collective requirement for the coreceptors GAS1, CDO, and BOC in SHH pathway function. Dev Cell. 2011. 20: 775-87
7. Angerer LM, Yaguchi S, Angerer RC, Burke RD. The evolution of nervous system patterning: Insights from sea urchin development. Development. 2011. 138: 3613-23
8. Ardern-Holmes S, Fisher G, North K. Neurofibromatosis type 2: Presentation, major complications, and management, with a focus on the pediatric age group. J Child Neurol. 2016. 32: 9-22
9. Attisano L, Wrana JL. Signal transduction by the TGF-beta superfamily. Science. 2002. 296: 1646-7
10. Augsburger A, Schuchardt A, Hoskins S, Dodd J, Butler S. BMPs as mediators of roof plate repulsion of commissural neurons. Neuron. 1999. 24: 127-41
11. Bagri A, Cheng HJ, Yaron A, Pleasure SJ, Tessier-Lavigne M. Stereotyped pruning of long hippocampal axon branches triggered by retraction inducers of the semaphorin family. Cell. 2003. 113: 285-99
12. Baron M. Induction of embryonic hematopoietic and endothelial stem/progenitor cells by hedgehog-mediated signals. Differentiation. 2001. 68: 175-85
13. Bayrak-Toydemir P, Mao R, Lewin S, McDonald J. Hereditary hemorrhagic telangiectasia: An overview of diagnosis and management in the molecular era for clinicians. Genet Med. 2004. 6: 175-91
14. Berg JN, Gallione CJ, Stenzel TT, Johnson DW, Allen WP, Schwartz CE. The activin receptor-like kinase 1 gene: Genomic structure and mutations in hereditary hemorrhagic telangiectasia type 2. Am J Hum Genet. 1997. 61: 60-7
15. Blockus H, Chedotal A. Slit-Robo signaling. Development. 2016. 143: 3037-44
16. Bourikas D, Pekarik V, Baeriswyl T, Grunditz A, Sadhu R, Nardo M. Sonic hedgehog guides commissural axons along the longitudinal axis of the spinal cord. Nat Neurosci. 2005. 8: 297-304
17. Bouvree K, Larrivee B, Lv X, Yuan L, DeLafarge B, Freitas C. Netrin-1 inhibits sprouting angiogenesis in developing avian embryos. Dev Biol. 2008. 318: 172-83
18. Briscoe J, Chen Y, Jessell TM, Struhl G. A hedgehog-insensitive form of patched provides evidence for direct long-range morphogen activity of sonic hedgehog in the neural tube. Mol Cell. 2001. 7: 1279-91
19. Brodhun M, Stahn V, Harder A. [Pathogenesis and molecular pathology of vestibular schwannoma]. HNO. 2017. 65: 362-72
20. Bruna A, Darken RS, Rojo F, Ocana A, Penuelas S, Arias A. High TGFbeta-Smad activity confers poor prognosis in glioma patients and promotes cell proliferation depending on the methylation of the PDGF-B gene. Cancer Cell. 2007. 11: 147-60
21. Butler SJ, Dodd J. A role for BMP heterodimers in roof plate-mediated repulsion of commissural axons. Neuron. 2003. 38: 389-401
22. Carmeliet P, Tessier-Lavigne M. Common mechanisms of nerve and blood vessel wiring. Nature. 2005. 436: 193-200
23. Cassavaugh J, Lounsbury KM. Hypoxia-mediated biological control. J Cell Biochem. 2011. 112: 735-44
24. Cattelino A, Liebner S, Gallini R, Zanetti A, Balconi G, Corsi A. The conditional inactivation of the beta-catenin gene in endothelial cells causes a defective vascular pattern and increased vascular fragility. J Cell Biol. 2003. 162: 1111-22
25. Caye-Thomasen P, Werther K, Nalla A, Bog-Hansen TC, Nielsen HJ, Stangerup SE. VEGF and VEGF receptor-1 concentration in vestibular schwannoma homogenates correlates to tumor growth rate. Otol Neurotol. 2005. 26: 98-101
26. Charron F, Stein E, Jeong J, McMahon AP, Tessier-Lavigne M. The morphogen sonic hedgehog is an axonal chemoattractant that collaborates with netrin-1 in midline axon guidance. Cell. 2003. 113: 11-23
27. Chédotal A. Slits and their receptors. Adv Exp Med Biol. 2007. 621: 65-80
28. Chinchilla P, Xiao L, Kazanietz MG, Riobo NA. Hedgehog proteins activate pro-angiogenic responses in endothelial cells through non-canonical signaling pathways. Cell Cycle. 2010. 9: 570-9
29. Clevers H, Nusse R. Wnt/beta-catenin signaling and disease. Cell. 2012. 149: 1192-205
30. Corada M, Nyqvist D, Orsenigo F, Caprini A, Giampietro C, Taketo MM. The Wnt/beta-catenin pathway modulates vascular remodeling and specification by upregulating Dll4/Notch signaling. Dev Cell. 2010. 18: 938-49
31. Curto M, Cole BK, Lallemand D, Liu CH, McClatchey AI. Contact-dependent inhibition of EGFR signaling by Nf2/Merlin. J Cell Biol. 2007. 177: 893-903
32. Daneman R, Agalliu D, Zhou L, Kuhnert F, Kuo CJ, Barres BA. Wnt/beta-catenin signaling is required for CNS, but not non-CNS, angiogenesis. Proc Natl Acad Sci U S A. 2009. 106: 641-6
33. Dessaud E, McMahon AP, Briscoe J. Pattern formation in the vertebrate neural tube: A sonic hedgehog morphogen-regulated transcriptional network. Development. 2008. 135: 2489-503
34. Dickson BJ. Molecular mechanisms of axon guidance. Science. 2002. 298: 1959-64
35. Dickson BJ, Gilestro GF. Regulation of commissural axon pathfinding by slit and its Robo receptors. Annu Rev Cell Dev Biol. 2006. 22: 651-5
36. Dickson BJ, Zou Y. Navigating intermediate targets: The nervous system midline. Cold Spring Harb Perspect Biol. 2010. 2: a002055-
37. Dieterich LC, Mellberg S, Langenkamp E, Zhang L, Zieba A, Salomaki H. Transcriptional profiling of human glioblastoma vessels indicates a key role of VEGF-A and TGFbeta2 in vascular abnormalization. J Pathol. 2012. 228: 378-90
38. Ducatman BS, Scheithauer BW, Piepgras DG, Reiman HM, Ilstrup DM. Malignant peripheral nerve sheath tumors. A clinicopathologic study of 120 cases. Cancer. 1986. 57: 2006-21
39. Dupuis-Girod S, Ginon I, Saurin JC, Marion D, Guillot E, Decullier E. Bevacizumab in patients with hereditary hemorrhagic telangiectasia and severe hepatic vascular malformations and high cardiac output. JAMA. 2012. 307: 948-55
40. Enomoto S, Mitsui K, Kawamura T, Iwanari H, Daigo K, Horiuchi K. Suppression of Slit2/Robo1 mediated HUVEC migration by Robo4. Biochem Biophys Res Commun. 2016. 469: 797-802
41. Evans TA, Bashaw GJ. Slit/Robo-mediated axon guidance in Tribolium and Drosophila: Divergent genetic programs build insect nervous systems. Dev Biol. 2012. 363: 266-78
42. Fabre PJ, Shimogori T, Charron F. Segregation of ipsilateral retinal ganglion cell axons at the optic chiasm requires the Shh receptor Boc. J Neurosci. 2010. 30: 266-75
43. Fazeli A, Dickinson SL, Hermiston ML, Tighe RV, Steen RG, Small CG. Phenotype of mice lacking functional Deleted in colorectal cancer (Dcc) gene. Nature. 1997. 386: 796-804
44. Fearnley GW, Smith GA, Abdul-Zani I, Yuldasheva N, Mughal NA, Homer-Vanniasinkam S. VEGF-A isoforms program differential VEGFR2 signal transduction, trafficking and proteolysis. Biol Open. 2016. 5: 571-83
45. Feldheim DA, Vanderhaeghen P, Hansen MJ, Frisen J, Lu Q, Barbacid M. Topographic guidance labels in a sensory projection to the forebrain. Neuron. 1998. 21: 1303-13
46. Fenstermaker AG, Prasad AA, Bechara A, Adolfs Y, Tissir F, Goffinet A. Wnt/planar cell polarity signaling controls the anterior-posterior organization of monoaminergic axons in the brainstem. J Neurosci. 2010. 30: 16053-64
47. Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003. 9: 669-76
48. Folkman J. Tumor angiogenesis: Therapeutic implications. N Engl J Med. 1971. 285: 1182-6
49. Folkman J, Klagsbrun M. Angiogenic factors. Science. 1987. 235: 442-7
50. Fong B, Barkhoudarian G, Pezeshkian P, Parsa AT, Gopen Q, Yang I. The molecular biology and novel treatments of vestibular schwannomas. J Neurosurg. 2011. 115: 906-14
51. Fujisawa H. Discovery of semaphorin receptors, neuropilin and plexin, and their functions in neural development. J Neurobiol. 2004. 59: 24-33
52. Fulling KH, Garcia DM. Anaplastic astrocytoma of the adult cerebrum. Prognostic value of histologic features. Cancer. 1985. 55: 928-931
53. Fuwa I, Wada H, Matsumoto T. [Recurrence of AVM after disappearing on postoperative angiography-report of two cases]. No Shinkei Geka. 1988. 16: 887-91
54. Gabriel EM, Sampson JH, Wilkins RH. Recurrence of a cerebral arteriovenous malformation after surgical excision. Case report. J Neurosurg. 1996. 84: 879-82
55. Gaengel K, Genove G, Armulik A, Betsholtz C. Endothelial-mural cell signaling in vascular development and angiogenesis. Arterioscler Thromb Vasc Biol. 2009. 29: 630-8
56. Gao PP, Yue Y, Zhang JH, Cerretti DP, Levitt P, Zhou R. Regulation of thalamic neurite outgrowth by the Eph ligand ephrin-A5: Implications in the development of thalamocortical projections. Proc Natl Acad Sci U S A. 1998. 95: 5329-34
57. Gao PP, Zhang JH, Yokoyama M, Racey B, Dreyfus CF, Black IB. Regulation of topographic projection in the brain: Elf-1 in the hippocamposeptal system. Proc Natl Acad Sci U S A. 1996. 93: 11161-6
58. Gerhardt H, Golding M, Fruttiger M, Ruhrberg C, Lundkvist A, Abramsson A. VEGF guides angiogenic sprouting utilizing endothelial tip cell filopodia. J Cell Biol. 2003. 161: 1163-77
59. Gittenberger-de Groot AC, DeRuiter MC, Bergwerff M, Poelmann RE. Smooth muscle cell origin and its relation to heterogeneity in development and disease. Arterioscler Thromb Vasc Biol. 1999. 19: 1589-94
60. Gordon L, Mansh M, Kinsman H, Morris AR. Xenopus sonic hedgehog guides retinal axons along the optic tract. Dev Dyn. 2010. 239: 2921-32
61. Grunwald IC, Korte M, Wolfer D, Wilkinson GA, Unsicker K, Lipp HP. Kinase-independent requirement of EphB2 receptors in hippocampal synaptic plasticity. Neuron. 2001. 32: 1027-40
62. Hammond R, Blaess S, Abeliovich A. Sonic hedgehog is a chemoattractant for midbrain dopaminergic axons. PLoS One. 2009. 4: e7007-
63. Hanna A, Shevde LA. Hedgehog signaling: Modulation of cancer properies and tumor mircroenvironment. Mol Cancer. 2016. 15: 24-
64. Hashimoto T, Wen G, Lawton MT, Boudreau NJ, Bollen AW, Yang GY. Abnormal expression of matrix metalloproteinases and tissue inhibitors of metalloproteinases in brain arteriovenous malformations. Stroke. 2003. 34: 925-31
65. Hellstrom M, Phng LK, Gerhardt H. VEGF and Notch signaling: The yin and yang of angiogenic sprouting. Cell Adh Migr. 2007. 1: 133-6
66. Helmbacher F, Schneider-Maunoury S, Topilko P, Tiret L, Charnay P. Targeting of the EphA4 tyrosine kinase receptor affects dorsal/ventral pathfinding of limb motor axons. Development. 2000. 127: 3313-24
67. Henriquez JP, Osses N. Editorial: Morphogens in the wiring of the nervous system. Front Cell Neurosci. 2015. 9: 502-
68. Herbert SP, Stainier DY. Molecular control of endothelial cell behaviour during blood vessel morphogenesis. Nat Rev Mol Cell Biol. 2011. 12: 551-64
69. Herzog Y, Kalcheim C, Kahane N, Reshef R, Neufeld G. Differential expression of neuropilin-1 and neuropilin-2 in arteries and veins. Mech Dev. 2001. 109: 115-9
70. Higuchi M, Bitoh S, Hasegawa H, Obashi J, Hiraga S. [Marked growth of arteriovenous malformation 19 years after resection: A case report]. No Shinkei Geka. 1991. 19: 75-8
71. Hughes SC, Fehon RG. Understanding ERM proteins-the awesome power of genetics finally brought to bear. Curr Opin Cell Biol. 2007. 19: 51-6
72. Hutchins BI, Li L, Kalil K. Wnt/calcium signaling mediates axon growth and guidance in the developing corpus callosum. Dev Neurobiol. 2011. 71: 269-83
73. Imayoshi I, Kageyama R. The role of Notch signaling in adult neurogenesis. Mol Neurobiol. 2011. 44: 7-12
74. Ingham PW, McMahon AP. Hedgehog signaling in animal development: Paradigms and principles. Genes Dev. 2001. 15: 3059-87
75. Izzi L, Levesque M, Morin S, Laniel D, Wilkes BC, Mille F. Boc and Gas1 each form distinct Shh receptor complexes with Ptch1 and are required for Shh-mediated cell proliferation. Dev Cell. 2011. 20: 788-801
76. Jellinger K. Vascular malformations of the central nervous system: A morphological overview. Neurosurg Rev. 1986. 9: 177-216
77. Jensen RL. Brain tumor hypoxia: Tumorigenesis, angiogenesis, imaging, pseudoprogression, and as a therapeutic target. J Neurooncol. 2009. 92: 317-35
78. Jessell TM. Neuronal specification in the spinal cord: Inductive signals and transcriptional codes. Nat Rev Genet. 2000. 1: 20-9
79. Jones CA, Nishiya N, London NR, Zhu W, Sorensen LK, Chan AC. Slit2-Robo4 signalling promotes vascular stability by blocking Arf6 activity. Nat Cell Biol. 2009. 11: 1325-31
80. Kageyama R, Ohtsuka T, Shimojo H, Imayoshi I. Dynamic regulation of Notch signaling in neural progenitor cells. Curr Opin Cell Biol. 2009. 21: 733-40
81. Kania A, Klein R. Mechanisms of ephrin-Eph signalling in development, physiology and disease. Nat Rev Mol Cell Biol. 2016. 17: 240-56
82. Kao TJ, Law C, Kania A. Eph and ephrin signaling: Lessons learned from spinal motor neurons. Semin Cell Dev Biol. 2012. 23: 83-91
83. Kawasaki T, Kitsukawa T, Bekku Y, Matsuda Y, Sanbo M, Yagi T. A requirement for neuropilin-1 in embryonic vessel formation. Development. 1999. 126: 4895-902
84. Keeble TR, Halford MM, Seaman C, Kee N, Macheda M, Anderson RB. The Wnt receptor Ryk is required for Wnt5a-mediated axon guidance on the contralateral side of the corpus callosum. J Neurosci. 2006. 26: 5840-8
85. Kennedy TE, Serafini T, de la Torre JR, Tessier-Lavigne M. Netrins are diffusible chemotropic factors for commissural axons in the embryonic spinal cord. Cell. 1994. 78: 425-35
86. Kidd T, Brose K, Mitchell KJ, Fetter RD, Tessier-Lavigne M, Goodman CS. Roundabout controls axon crossing of the CNS midline and defines a novel subfamily of evolutionarily conserved guidance receptors. Cell. 1998. 92: 205-15
87. Kissil JL, Wilker EW, Johnson KC, Eckman MS, Yaffe MB, Jacks T. Merlin, the product of the Nf2 tumor suppressor gene, is an inhibitor of the p21-activated kinase, Pak1. Mol Cell. 2003. 12: 841-9
88. Kondziolka D, Humphreys RP, Hoffman HJ, Hendrick EB, Drake JM. Arteriovenous malformations of the brain in children: A forty year experience. Can J Neurol Sci. 1992. 19: 40-5
89. Koropouli E, Kolodkin AL. Semaphorins and the dynamic regulation of synapse assembly, refinement, and function. Curr Opin Neurobiol. 2014. 27: 1-7
90. Larrivee B, Freitas C, Trombe M, Lv X, Delafarge B, Yuan L. Activation of the UNC5B receptor by Netrin-1 inhibits sprouting angiogenesis. Genes Dev. 2007. 21: 2433-47
91. Lee CZ, Yao JS, Huang Y, Zhai W, Liu W, Guglielmo BJ. Dose-response effect of tetracyclines on cerebral matrix metalloproteinase-9 after vascular endothelial growth factor hyperstimulation. J Cereb Blood Flow Metab. 2006. 26: 1157-64
92. Lee DC, Sohn HA, Park ZY, Oh S, Kang YK, Lee KM. A lactate-induced response to hypoxia. Cell. 2015. 161: 595-609
93. Lee JW, Bae SH, Jeong JW, Kim SH, Kim KW. Hypoxia-inducible factor (HIF-1) alpha: Its protein stability and biological functions. Exp Mol Med. 2004. 36: 1-12
94. Lee KJ, Mendelsohn M, Jessell TM. Neuronal patterning by BMPs: A requirement for GDF7 in the generation of a discrete class of commissural interneurons in the mouse spinal cord. Genes Dev. 1998. 12: 3394-407
95. Leslie JD, Ariza-McNaughton L, Bermange AL, McAdow R, Johnson SL, Lewis J. Endothelial signalling by the Notch ligand Delta-like 4 restricts angiogenesis. Development. 2007. 134: 839-44
96. Li W, Cooper J, Zhou L, Yang C, Erdjument-Bromage H, Zagzag D. Merlin/NF2 loss-driven tumorigenesis linked to CRL4(DCAF1)-mediated inhibition of the hippo pathway kinases Lats1 and 2 in the nucleus. Cancer Cell. 2014. 26: 48-60
97. Li W, You L, Cooper J, Schiavon G, Pepe-Caprio A, Zhou L. Merlin/NF2 suppresses tumorigenesis by inhibiting the E3 ubiquitin ligase CRL4(DCAF1) in the nucleus. Cell. 2010. 140: 477-90
98. Liebner S, Corada M, Bangsow T, Babbage J, Taddei A, Czupalla CJ. Wnt/beta-catenin signaling controls development of the blood-brain barrier. J Cell Biol. 2008. 183: 409-17
99. Lim M, Cheshier S, Steinberg GK. New vessel formation in the central nervous system during tumor growth, vascular malformations, and Moyamoya. Curr Neurovasc Res. 2006. 3: 237-45
100. Liu XB, Low LK, Jones EG, Cheng HJ. Stereotyped axon pruning via plexin signaling is associated with synaptic complex elimination in the hippocampus. J Neurosci. 2005. 25: 9124-34
101. Liu Y, Shi J, Lu CC, Wang ZB, Lyuksyutova AI, Song XJ. Ryk-mediated Wnt repulsion regulates posterior-directed growth of corticospinal tract. Nat Neurosci. 2005. 8: 1151-9
102. Lowe CJ, Wu M, Salic A, Evans L, Lander E, Stange-Thomann N. Anteroposterior patterning in hemichordates and the origins of the chordate nervous system. Cell. 2003. 113: 853-65
103. Lu X, Le Noble F, Yuan L, Jiang Q, De Lafarge B, Sugiyama D. The netrin receptor UNC5B mediates guidance events controlling morphogenesis of the vascular system. Nature. 2004. 432: 179-86
104. Luria V, Krawchuk D, Jessell TM, Laufer E, Kania A. Specification of motor axon trajectory by ephrin-B: EphB signaling: Symmetrical control of axonal patterning in the developing limb. Neuron. 2008. 60: 1039-53
105. Lyuksyutova AI, Lu CC, Milanesio N, King LA, Guo N, Wang Y. Anterior-posterior guidance of commissural axons by Wnt-frizzled signaling. Science. 2003. 302: 1984-8
106. MacCollin M, Ramesh V, Jacoby LB, Louis DN, Rubio MP, Pulaski K. Mutational analysis of patients with neurofibromatosis 2. Am J Hum Genet. 1994. 55: 314-20
107. Mahajan S, Athale CA. Spatial and temporal sensing limits of microtubule polarization in neuronal growth cones by intracellular gradients and forces. Biophys J. 2012. 103: 2432-45
108. Maisonpierre PC, Suri C, Jones PF, Bartunkova S, Wiegand SJ, Radziejewski C. Angiopoietin-2, a natural antagonist for Tie2 that disrupts in vivo angiogenesis. Science. 1997. 277: 55-60
109. Massague J. TGFbeta in Cancer. Cell. 2008. 134: 215-30
110. Maurel P, Salzer JL. Axonal regulation of Schwann cell proliferation and survival and the initial events of myelination requires PI 3-kinase activity. J Neurosci. 2000. 20: 4635-45
111. Mautner V, Nguyen R, Friedrich R, Kutta H, Fuensterer C, Hagel C. Bevacizumab induces regression of vestibular schwannomas leading to improved hearing in NF2 patients. Onkologie. 2010. 33: 57-
112. Maxwell PH, Wiesener MS, Chang GW, Clifford SC, Vaux EC, Cockman ME. The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature. 1999. 399: 271-5
113. Moftakhar P, Hauptman JS, Malkasian D, Martin NA. Cerebral arteriovenous malformations. Part 1: Cellular and molecular biology. Neurosurg Focus. 2009. 26: E10-
114. Moftakhar P, Hauptman JS, Malkasian D, Martin NA. Cerebral arteriovenous malformations. Part 2: Physiology. Neurosurg Focus. 2009. 26: E11-
115. Mukouyama YS, Shin D, Britsch S, Taniguchi M, Anderson DJ. Sensory nerves determine the pattern of arterial differentiation and blood vessel branching in the skin. Cell. 2002. 109: 693-705
116. Neff BA, Welling DB, Akhmametyeva E, Chang LS. The molecular biology of vestibular schwannomas: Dissecting the pathogenic process at the molecular level. Otol Neurotol. 2006. 27: 197-208
117. Neufeld G, Kessler O. The semaphorins: Versatile regulators of tumour progression and tumour angiogenesis. Nat Rev Cancer. 2008. 8: 632-45
118. Niehrs C. The complex world of WNT receptor signalling. Nat Rev Mol Cell Biol. 2012. 13: 767-79
119. Noguera-Troise I, Daly C, Papadopoulos NJ, Coetzee S, Boland P, Gale NW. Blockade of Dll4 inhibits tumour growth by promoting non-productive angiogenesis. Nature. 2006. 444: 1032-7
120. Nolt MJ, Lin Y, Hruska M, Murphy J, Sheffler-Colins SI, Kayser MS. EphB controls NMDA receptor function and synaptic targeting in a subunit-specific manner. J Neurosci. 2011. 31: 5353-64
121. Norris AD, Lundquist EA. UNC-6/netrin and its receptors UNC-5 and UNC-40/DCC modulate growth cone protrusion in vivo in C. elegans. Development. 2011. 138: 4433-42
122. Nusslein-Volhard C, Wieschaus E. Mutations affecting segment number and polarity in Drosophila. Nature. 1980. 287: 795-801
123. O’Donnell MP, Bashaw GJ. Distinct functional domains of the Abelson tyrosine kinase control axon guidance responses to Netrin and Slit to regulate the assembly of neural circuits. Development. 2013. 140: 2724-33
124. Palmer A, Klein R. Multiple roles of ephrins in morphogenesis, neuronal networking, and brain function. Genes Dev. 2003. 17: 1429-50
125. Pardali E, Goumans MJ, ten Dijke P. Signaling by members of the TGF-beta family in vascular morphogenesis and disease. Trends Cell Biol. 2010. 20: 556-67
126. Park KM, Gerecht S. Harnessing developmental processes for vascular engineering and regeneration. Development. 2014. 141: 2760-9
127. Park KW, Morrison CM, Sorensen LK, Jones CA, Rao Y, Chien CB. Robo4 is a vascular-specific receptor that inhibits endothelial migration. Dev Biol. 2003. 261: 251-67
128. Parkash J, Messina A, Langlet F, Cimino I, Loyens A, Mazur D. Semaphorin7A regulates neuroglial plasticity in the adult hypothalamic median eminence. Nat Commun. 2015. 6: 6385-
129. Phng LK, Potente M, Leslie JD, Babbage J, Nyqvist D, Lobov I. Nrarp coordinates endothelial Notch and Wnt signaling to control vessel density in angiogenesis. Dev Cell. 2009. 16: 70-82
130. Plotkin SR, Stemmer-Rachamimov AO, Barker FG, 2nd , Halpin C, Padera TP, Tyrrell A. Hearing improvement after bevacizumab in patients with neurofibromatosis type 2. N Engl J Med. 2009. 361: 358-67
131. Porter PJ, Willinsky RA, Harper W, Wallace MC. Cerebral cavernous malformations: Natural history and prognosis after clinical deterioration with or without hemorrhage. J Neurosurg. 1997. 87: 190-7
132. Potente M, Gerhardt H, Carmeliet P. Basic and therapeutic aspects of angiogenesis. Cell. 2011. 146: 873-87
133. Putman CM, Chaloupka JC, Fulbright RK, Awad IA, White RI, Fayad PB. Exceptional multiplicity of cerebral arteriovenous malformations associated with hereditary hemorrhagic telangiectasia (Osler-Weber-Rendu syndrome). AJNR Am J Neuroradiol. 1996. 17: 1733-42
134. Pyo R, Lee JK, Shipley JM, Curci JA, Mao D, Ziporin SJ. Targeted gene disruption of matrix metalloproteinase-9 (gelatinase B) suppresses development of experimental abdominal aortic aneurysms. J Clin Invest. 2000. 105: 1641-9
135. Rangel-Castilla L, Russin JJ, Martinez-Del-Campo E, Soriano-Baron H, Spetzler RF, Nakaji P. Molecular and cellular biology of cerebral arteriovenous malformations: A review of current concepts and future trends in treatment. Neurosurg Focus. 2014. 37: E1-
136. Raper J, Mason C. Cellular strategies of axonal pathfinding. Cold Spring Harb Perspect Biol. 2010. 2: a001933-
137. Ridgway J, Zhang G, Wu Y, Stawicki S, Liang WC, Chanthery Y. Inhibition of Dll4 signalling inhibits tumour growth by deregulating angiogenesis. Nature. 2006. 444: 1083-7
138. Risau W, Flamme I. Vasculogenesis. Annu Rev Cell Dev Biol. 1995. 11: 73-91
139. Roelink H, Porter JA, Chiang C, Tanabe Y, Chang DT, Beachy PA. Floor plate and motor neuron induction by different concentrations of the amino-terminal cleavage product of sonic hedgehog autoproteolysis. Cell. 1995. 81: 445-55
140. Roth L, Prahst C, Ruckdeschel T, Savant S, Westrom S, Fantin A. Neuropilin-1 mediates vascular permeability independently of vascular endothelial growth factor receptor-2 activation. Sci Signal. 2016. 9: ra42-
141. Rothbart D, Awad IA, Lee J, Kim J, Harbaugh R, Criscuolo GR. Expression of angiogenic factors and structural proteins in central nervous system vascular malformations. Neurosurgery. 1996. 38: 915-24
142. Rouleau GA, Merel P, Lutchman M, Sanson M, Zucman J, Marineau C. Alteration in a new gene encoding a putative membrane-organizing protein causes neuro-fibromatosis type 2. Nature. 1993. 363: 515-21
143. Ruhrberg C, Gerhardt H, Golding M, Watson R, Ioannidou S, Fujisawa H. Spatially restricted patterning cues provided by heparin-binding VEGF-A control blood vessel branching morphogenesis. Genes Dev. 2002. 16: 2684-98
144. Ruiz de Almodovar C, Fabre PJ, Knevels E, Coulon C, Segura I, Haddick PC. VEGF mediates commissural axon chemoattraction through its receptor Flk1. Neuron. 2011. 70: 966-78
145. Sanchez-Camacho C, Bovolenta P. Autonomous and non-autonomous Shh signalling mediate the in vivo growth and guidance of mouse retinal ganglion cell axons. Development. 2008. 135: 3531-41
146. Sanvoranart T, Supokawej A, Kheolamai P, Y UP, Poungvarin N, Sathornsumetee S. Targeting Netrin-1 in glioblastoma stem-like cells inhibits growth, invasion, and angiogenesis. Tumour Biol. 2016. 37: 14949-60
147. Schmitt AM, Shi J, Wolf AM, Lu CC, King LA, Zou Y. Wnt-Ryk signalling mediates medial-lateral retinotectal topographic mapping. Nature. 2006. 439: 31-7
148. Semenza GL. Targeting HIF-1 for cancer therapy. Nat Rev Cancer. 2003. 3: 721-32
149. Serafini T, Colamarino SA, Leonardo ED, Wang H, Beddington R, Skarnes WC. Netrin-1 is required for commissural axon guidance in the developing vertebrate nervous system. Cell. 1996. 87: 1001-14
150. Shaw RJ, Paez JG, Curto M, Yaktine A, Pruitt WM, Saotome I. The Nf2 tumor suppressor, merlin, functions in Rac-dependent signaling. Dev Cell. 2001. 1: 63-72
151. Shenkar R, Elliott JP, Diener K, Gault J, Hu LJ, Cohrs RJ. Differential gene expression in human cerebrovascular malformations. Neurosurgery. 2003. 52: 465-77
152. Shih AH, Holland EC. Platelet-derived growth factor (PDGF) and glial tumorigenesis. Cancer Lett. 2006. 232: 139-47
153. Siekmann AF, Lawson ND. Notch signalling limits angiogenic cell behaviour in developing zebrafish arteries. Nature. 2007. 445: 781-4
154. Simon MC. The Hypoxia Response Pathways - Hats Off!. N Engl J Med. 2016. 375: 1687-9
155. Song H, Ming G, He Z, Lehmann M, McKerracher L, Tessier-Lavigne M. Conversion of neuronal growth cone responses from repulsion to attraction by cyclic nucleotides. Science. 1998. 281: 1515-8
156. Song H, Poo M. The cell biology of neuronal navigation. Nat Cell Biol. 2001. 3: E81-8
157. Sonoda Y, Kanamori M, Deen DF, Cheng SY, Berger MS, Pieper RO. Overexpression of vascular endothelial growth factor isoforms drives oxygenation and growth but not progression to glioblastoma multiforme in a human model of gliomagenesis. Cancer Res. 2003. 63: 1962-8
158. Stein E, Tessier-Lavigne M. Hierarchical organization of guidance receptors: Silencing of netrin attraction by slit through a Robo/DCC receptor complex. Science. 2001. 291: 1928-38
159. Stenman JM, Rajagopal J, Carroll TJ, Ishibashi M, McMahon J, McMahon AP. Canonical Wnt signaling regulates organ-specific assembly and differentiation of CNS vasculature. Science. 2008. 322: 1247-50
160. Sternlicht MD, Werb Z. How matrix metalloproteinases regulate cell behavior. Annu Rev Cell Dev Biol. 2001. 17: 463-516
161. Sturiale CL, Puca A, Sebastiani P, Gatto I, Albanese A, Di Rocco C. Single nucleotide polymorphisms associated with sporadic brain arteriovenous malformations: Where do we stand?. Brain. 2013. 136: 665-81
162. Tate MC, Aghi MK. Biology of angiogenesis and invasion in glioma. Neurotherapeutics. 2009. 6: 447-57
163. Tessier-Lavigne M, Goodman CS. The molecular biology of axon guidance. Science. 1996. 274: 1123-33
164. Thomas JM, Surendran S, Abraham M, Rajavelu A, Kartha CC. Genetic and epigenetic mechanisms in the development of arteriovenous malformations in the brain. Clin Epigenetics. 2016. 8: 78-
165. Tu T, Zhang C, Yan H, Luo Y, Kong R, Wen P. CD146 acts as a novel receptor for netrin-1 in promoting angiogenesis and vascular development. Cell Res. 2015. 25: 275-87
166. Uesaka T, Shono T, Suzuki SO, Nakamizo A, Niiro H, Mizoguchi M. Expression of VEGF and its receptor genes in intracranial schwannomas. J Neurooncol. 2007. 83: 259-66
167. Vernimmen F. Vascular endothelial growth factor blockade: A potential new therapy in the management of cerebral arteriovenous malformations. J Med Hypotheses Ideas. 2014. 8: 57-61
168. Wang H, Charles PC, Wu Y, Ren R, Pi X, Moser M. Gene expression profile signatures indicate a role for Wnt signaling in endothelial commitment from embryonic stem cells. Circ Res. 2006. 98: 1331-9
169. Wang HU, Chen ZF, Anderson DJ. Molecular distinction and angiogenic interaction between embryonic arteries and veins revealed by ephrin-B2 and its receptor Eph-B4. Cell. 1998. 93: 741-53
170. Wilkinson DG. Multiple roles of EPH receptors and ephrins in neural development. Nat Rev Neurosci. 2001. 2: 155-64
171. Wilson BD, Ii M, Park KW, Suli A, Sorensen LK, Larrieu-Lahargue F. Netrins promote developmental and therapeutic angiogenesis. Science. 2006. 313: 640-4
172. Xin H, Zhong C, Nudleman E, Ferrara N. Evidence for Pro-angiogenic Functions of VEGF-Ax. Cell. 2016. 167: 275-84 e276
173. Yam PT, Kent CB, Morin S, Farmer WT, Alchini R, Lepelletier L. 14-3-3 proteins regulate a cell-intrinsic switch from sonic hedgehog-mediated commissural axon attraction to repulsion after midline crossing. Neuron. 2012. 76: 735-49
174. Yazdani U, Terman JR. The semaphorins. Genome Biol. 2006. 7: 211-
175. Zhang B, Dietrich UM, Geng JG, Bicknell R, Esko JD, Wang L. Repulsive axon guidance molecule Slit3 is a novel angiogenic factor. Blood. 2009. 114: 4300-9
176. Zhang G, Chen L, Sun K, Khan AA, Yan J, Liu H. Neuropilin-1 (NRP-1)/GIPC1 pathway mediates glioma progression. Tumour Biol. 2016. 37: 13777-88
177. Zhou Y, Gunput RA, Pasterkamp RJ. Semaphorin signaling: Progress made and promises ahead. Trends Biochem Sci. 2008. 33: 161-70