- Department of Surgery, University of Zimbabwe, College of Health Sciences, Harare, Zimbabwe
- Department of Neurological Surgery, School of Medicine and Public Health, University of Wisconsin, Madison, USA
Correspondence Address:
Luxwell Jokonya
Department of Surgery, University of Zimbabwe, College of Health Sciences, Harare, Zimbabwe
DOI:10.4103/sni.sni_423_17
Copyright: © 2018 Surgical Neurology International This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.How to cite this article: Luxwell Jokonya, Aaron Musara, Ignatius Ngene Esene, Kantenga Dieu Merci Kabulo, Charles Matumba Kabeya, Kazadi Kaluile Ntenga Kalangu. Prevalence of human immunodeficiency virus infection in brain glioma patients: Is the virus protective from gliomas?. 24-May-2018;9:103
How to cite this URL: Luxwell Jokonya, Aaron Musara, Ignatius Ngene Esene, Kantenga Dieu Merci Kabulo, Charles Matumba Kabeya, Kazadi Kaluile Ntenga Kalangu. Prevalence of human immunodeficiency virus infection in brain glioma patients: Is the virus protective from gliomas?. 24-May-2018;9:103. Available from: http://surgicalneurologyint.com/surgicalint-articles/prevalence-of-human-immunodeficiency-virus-infection-in-brain-glioma-patients-is-the-virus-protective-from-gliomas/
Abstract
Background:Human immunodeficiency virus/acquired immunodeficiency syndrome (HIV/AIDS) is associated with an increased prevalence of some malignancies. However, some observational studies have revealed an ever-decreasing prevalence of HIV in glioma patients. The relationship between HIV and brain gliomas has not been well established.
Methods:A cross-sectional study was carried out in sub-Sahara Africa, a high HIV prevalence setting, to determine the prevalence of HIV among all glioma patients over a 2-year period.
Results:A markedly reduced prevalence of HIV was found in glioma patients (8.3%) in comparison to the general population (14.3%). The presumably “antiglioma effect” of HIV and/or its treatment resulted in a 42% decrease in glioma occurrence in HIV positive patients compared to HIV negative individuals. Age and sex-adjusted prevalence were also lower among glioma patients with the protective effect observed more in younger patients and female sex.
Conclusion:Our results corroborate the protective effect of HIV positivity vis-à -vis gliomas. This “antiglioma effect” could be attributed to either the HIV, its treatment, or both. Future studies focused on this “effect” may help unveil better preventative and possible therapeutic avenues for gliomas.
Keywords: Brain tumors, glioma, human immunodeficiency virus, infections
INTRODUCTION
Human immunodeficiency virus (HIV) and acquired immunodeficiency syndrome (AIDS) has been associated with an increased prevalence of malignancy. Reduced immunity leading to the decreased surveillance of oncogenic elements and increased incidence of oncogenic opportunistic infections are among some of the reasons for such an increased incidence of malignancy.[
MATERIALS AND METHODS
A cross-sectional study was carried out in Zimbabwe. All patients who presented to Zimbabwe Hospitals and eventually had a histological diagnosis of a brain glioma between January 1, 2014 and December 31, 2015 were enrolled for the study. Patients whose initial presentation was outside the study period were excluded. Case definition of a brain glioma patient was as per World Health Organization (WHO) grading system 2007. It included all glial cell origin tumors: astrocytomas, ependymomas, oligodendrogliomas, and mixed glial cell tumors.
Liaison was constantly made with all the neurosurgeons in the country during the study such that information about any glioma patient seen was availed. In addition, liaison was also made with all the pathology laboratories in the country so that information on all brain biopsy results in the country were accessed. This allowed access to data on all patients who had brain tumors operated on in the country. The patients were managed as per the prevailing neurosurgical protocol and the study did not interfere with their standard management.
All patients eligible were contacted, and none declined consent. The patient or caregiver was interviewed and imaging and histology done were reviewed. Histological grading was done using the WHO grading system and immunohistochemistry was done for equivocal cases. If the patient did not have a recent (within 3 months) documented HIV test result, he/she was offered HIV counselling and testing. Those who were HIV positive would have cluster of differentiation 4 (CD4) counts done. The data collected were then analyzed.
A confidential data collection form was administered by the researchers. Data were then captured into Epi info ™ 7(The Epi info Software, Centers for Disease Control and Prevention “CDC” in Atlanta, Georgia, USA) for analysis. Data were then exported to Stata 13 (StataCorp, Texas, USA) for further analysis.
We analyzed our data making comparison to that of the Zimbabwe Health Survey[
RESULTS
A total of 60 patients were suspected of having brain gliomas during the study period. Forty-nine of these had biopsy done but only 48 cases (with a histologically proven glioma and known HIV status) were included in the final analysis. One had an unknown HIV status and was excluded. For the other 11 patients excluded in the subsequent analysis, the diagnosis of glioma was based on imaging studies only. The reasons for lack of biopsy were inoperability in diffuse pontine glioma (4), declined operation (3), demised before operation (2), too ill for operation (1), and lost to follow-up before biopsy (1).
The mean age [±standard deviation (SD)] of the study group (n = 48 patients) was 40.5 ± 23.3 years (min 3 years; max 87 years). Age distribution was bimodal with peaks at around 3 years and another between 40 and 50 years. There were more females, 54.1% (26/48), among the study participants. There was no significant difference in age distribution between males mean age (±SD) [38.6 ± 21.9 years] and females (±SD) [42.1 ± 24.8 years] (P = 0.6156). Eighty-five percent (n = 41) were black, 15% (n = 7) were whites. Urban settlers accounted for 85.4% (n = 41) of the patients, whereas 14.6% (n = 7) were from rural areas. The majority were from Harare (urban).
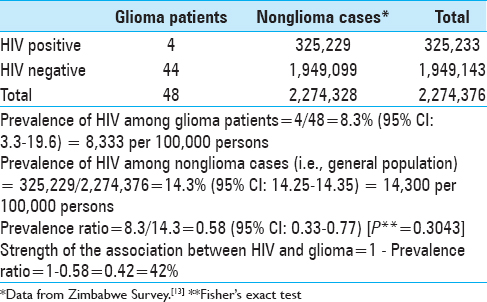
Four participants were HIV positive. Three (75%) were males and there was one female (25%). The median age of the four cases was 49.5 years (p25 = 38; p75 = 54) (min = 29, max = 57) [
The prevalence of HIV among glioma patients was 8.3% (95% CI: 3.3; 19.6) and among nonglioma cases was 14.3% (95% CI: 14.25; 14.35). This gave a prevalence ratio of 0.58 (95% CI: 0.33; 0.77). The strength of the association between HIV (exposure) and glioma (disease or outcome) was 42% implying the “antiglioma effect” of HIV and/or its treatment resulted in a 42% decrease in glioma occurrence in HIV positive patients compared to HIV negative individuals [
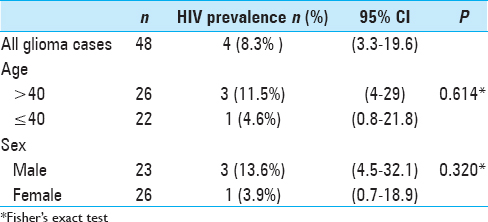
Age and sex-adjusted prevalence revealed lower proportions of HIV seropositivity among glioma patients compared to the general population. The prevalence was lower in younger patients (≤40 years) [1 case (4.6%)] and females [1 case (3.9%)]. Of the four cases with HIV positive serology, one patient not on antiretroviral therapy, one had a protease inhibitor in his regiment, whereas the other two did not have a protease inhibitor in their regiment [
DISCUSSION
Our study showed an 8.3% prevalence of HIV in glioma patients versus 14.3% in the general population.[
The distribution of gliomas in the country is evidently skewed. Majority of the glioma patients were from urban centers, which corresponds with a lower risk for HIV infection. The heterogeneous distribution of HIV rates is such that urban centers were at 14%, and Harare (where most glioma patients were from) 13% versus 8.3% for glioma patients.[
Epidermal growth factor has been found in increased amounts and has been shown to stimulate vascular endothelial growth factor (VEGF) production in glioblastoma, and plays a key role in the pathogenesis of glioblastoma.[
Furthermore, an inverse relationship between acute infections and the subsequent development of some cancers (such as meningiomas, melanomas, and gliomas) has been established. Overall risk reduction increased with frequency of infections, with febrile infections affording the greatest protection. However, chronic infections can be viewed as resulting from a failed immune response and an increasing number has been associated with an increased cancer risk.[
It is interesting to note that some glioma cells have an “anti-HIV effect.” A study demonstrated that some but not all glioma cells secrete inhibitory molecules to HIV infection that contribute to lowering HIV infection in the central nervous system in vivo.[
The possible presence of undiagnosed cases of gliomas in the general population could be a confounding factor questioning the internal validity of our study and rendering the general population not ideal as a comparison group. However, the low prevalence of gliomas in our setting dilutes the possible confounding effect. Among the glioma patients, the onset of HIV infection is difficult to determine, complicating the establishment of an association between HIV and glioma. However, our study provides evidence from Africa, the only continent where data was lacking on the relationship between HIV/AIDS and glioma prevalence to supplement those of the other continents.[
CONCLUSION
This study, done in a setting of high HIV prevalence, shows a prevalence of HIV in glioma patients that is markedly less than in the general population, notwithstanding that the sample size was small owing to the short duration of the study. Based on the prevailing status quo, it would make sense that any effort made at preventing gliomas would stand a better chance in fighting this menace. Perhaps, a more detailed exploration of how this “antiglioma” effect of HIV works may help unveil better preventative and possible therapeutic technologies.
Funding: No funding was received for this research.
Ethical approval: Authority to perform the study was obtained from the Joint Research and Ethics Committee. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent: Informed consent was obtained from all individual participants included in the study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Cedeno-Laurent F, Trujillo JR. Gliomas and brain lymphomas in HIV-1/AIDS patients: Reflections from a 20-year follow up in Mexico and Brazil. Microbiol Res (Pavia). 2011. 2: 11-
2. Chaudhry NS, Ahmad FU, Blieden C, Benveniste RJ. Brainstem anaplastic glioma in patients with AIDS: A case report and review of the literature. BMJ Case Rep 2013. 2013. p.
3. Cohen MS, Shaw GM, McMichael AJ, Haynes BF. Acute HIV-1 infection. N Engl J Med. 2011. 364: 1943-54
4. Gabizon A, Leibovich SJ, Goldman R. Contrasting effects of activated and nonactivated macrophages and macrophages from tumor-bearing mice on tumor growth in vivo. J Natl Cancer Inst. 1980. 65: 913-20
5. Goldman CK, Kim J, Wong WL, King V, Brock T, Gillespie GY. Epidermal growth factor stimulates vascular endothelial growth factor production by human malignant glioma cells: A model of glioblastoma multiforme pathophysiology. Mol Biol Cell. 1993. 4: 121-33
6. Hall JR, Short SC. Management of glioblastoma multiforme in HIV patients: A case series and review of published studies. Clin Oncol (R Coll Radiol). 2009. 21: 591-7
7. Hoption Cann SA, Van Netten JP, Van Netten C. Acute infections as a means of cancer prevention: Opposing effects to chronic infections?. Cancer Detect Prev. 2006. 30: 83-93
8. Hoque SA, Tanaka A, Islam S, Ahsan GU, Jinno-Oue A, Hoshino H. Suppression of HIV-1 infectivity by human glioma cells. AIDS Res Hum Retroviruses. 2016. 32: 480-8
9. Kerina D, Babill SP. HIV/AIDS: The Zimbabwean situation and trends. Am J Clin Med Res. 2013. 1: 15-22
10. Newcombe RG. Two-sided confidence intervals for the single proportion: Comparison of seven methods. Stat Med. 1998. 17: 857-72
11. Pore N, Gupta AK, Cerniglia GJ, Maity A. HIV protease inhibitors decrease VEGF/HIF-1alpha expression and angiogenesis in glioblastoma cells. Neoplasia. 2006. 8: 889-95
12. Stevanovic M, Sharpe F, Wolfe S, Pederson W, Hotchkiss R, Kozin S, Cohen M.editors. Acute infections. Green's Operative Hand Surge. New York: Elsevier Inc; 2010. p. 41-84
13. Last accessed on 2017 Aug 20].. Available from: https://searchworks.stanford.edu/view/11748462.