- Department of Neurosurgery, Government Medical College, Kota, Rajasthan, India
- Department of Neurosurgery, All India Institute of Medical Sciences, New Delhi, India
Correspondence Address:
Rajesh K. Meena
Department of Neurosurgery, All India Institute of Medical Sciences, New Delhi, India
DOI:10.4103/sni.sni_219_17
Copyright: © 2017 Surgical Neurology International This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Sachidanand Gautam, Rajesh K. Meena. Primary intracranial leiomyosarcoma presenting with massive peritumoral edema and mass effect: Case report and literature review. 20-Nov-2017;8:278
How to cite this URL: Sachidanand Gautam, Rajesh K. Meena. Primary intracranial leiomyosarcoma presenting with massive peritumoral edema and mass effect: Case report and literature review. 20-Nov-2017;8:278. Available from: http://surgicalneurologyint.com/surgicalint-articles/primary-intracranial-leiomyosarcoma-presenting-with-massive-peritumoral-edema-and-mass-effect-case-report-and-literature-review/
Abstract
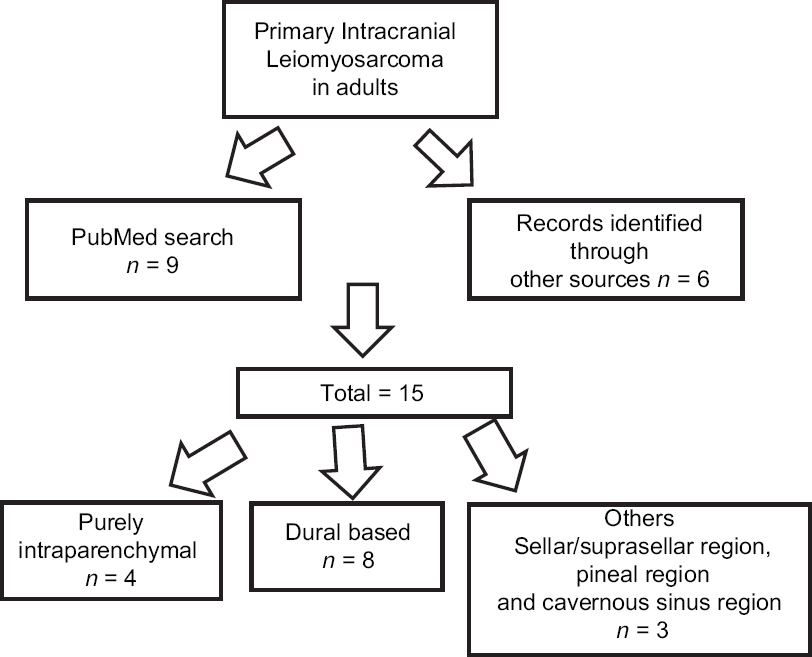
Background:Primary intracranial leiomyosarcomas (LMSs) are unusual tumors of the central nervous system (CNS) affecting all age groups, and are recently, becoming more prevalent in immunosuppressive conditions such as in patients with human immunodeficiency virus (HIV) infection. However, only a few CNS LMS case reports exist in the English literature, on the occurrence of this rare entity in immunocompetent adults. Even, rarer is a purely intraparenchymal occurrence without any dural attachment in afflicted individuals. To the best of our knowledge, only four such cases have been reported in the literature until now. None of these cases were associated with marked peritumoral brain edema (PTBE) and mass effect as seen in our case and falsely suggesting an underlying glioma.
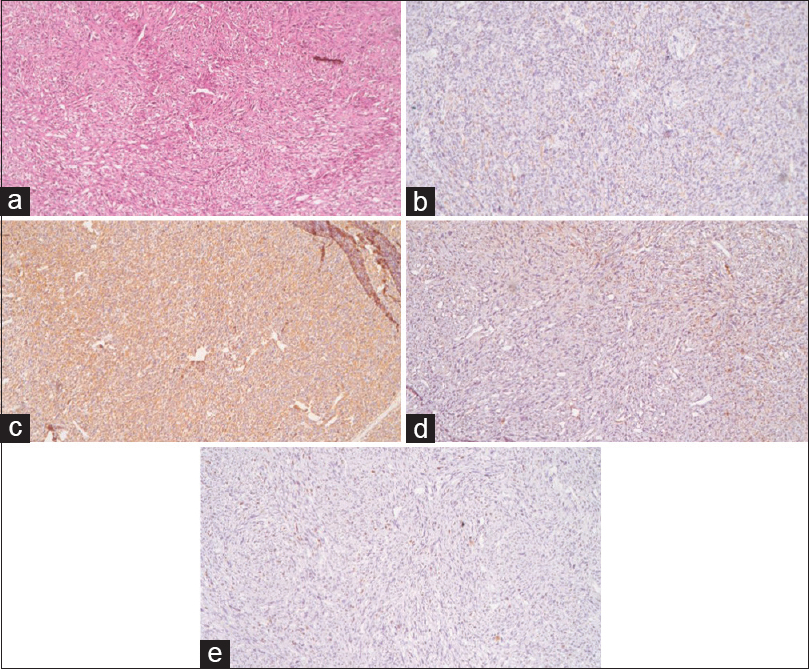
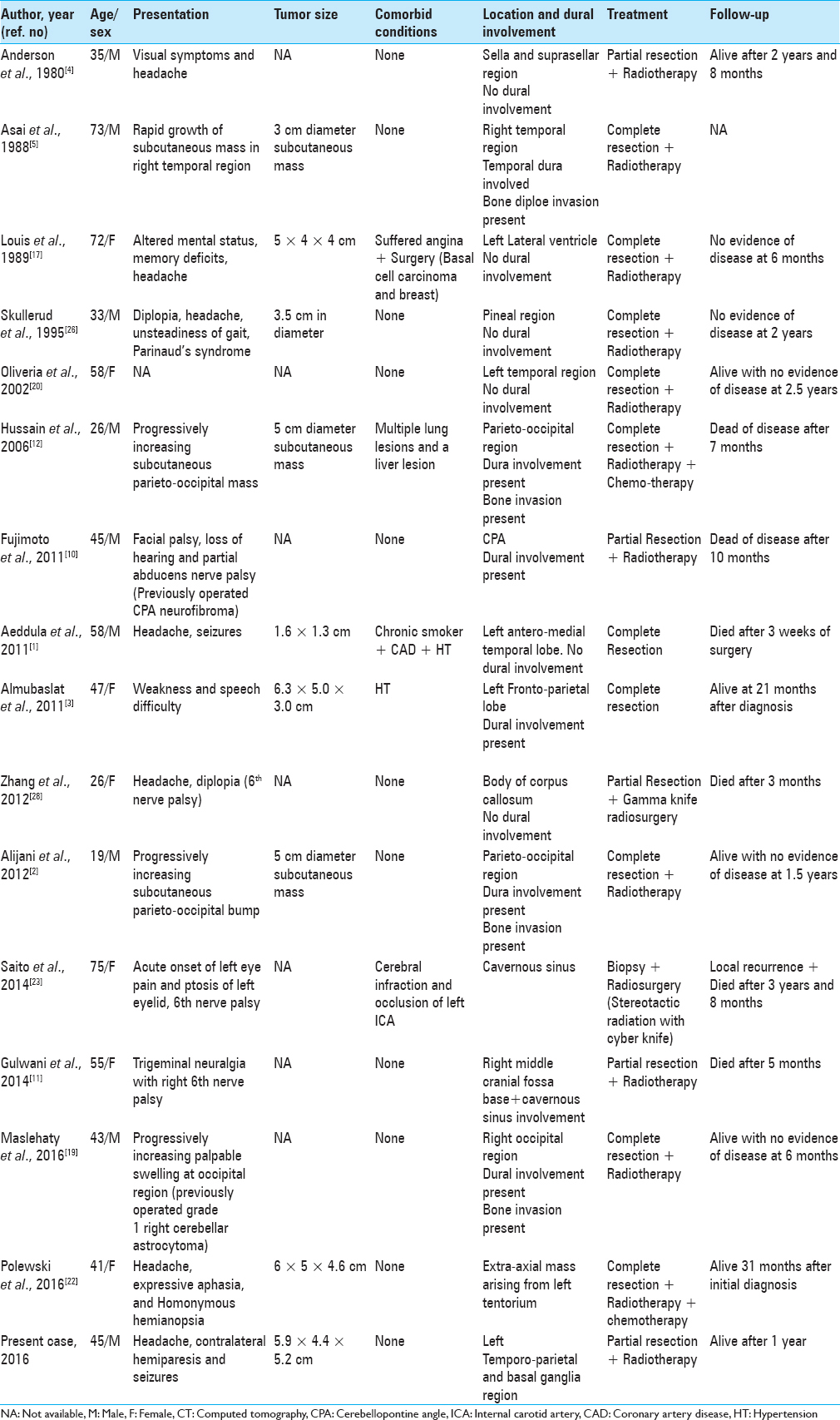
Case Description:A 45-year-old male patient, presented with headache, right-sided weakness and difficulties with speech over 4 months along with a single generalized tonic clonic seizure. Physical examination revealed mild to moderate papilledema, motor aphasia, and right-sided hemiparesis. Radiographic evaluation showed a large left temporo-parietal mass extending into the basal ganglia with intense heterogeneous contrast enhancement. There was marked perilesional edema and mass effect with midline shift. The patient underwent a left temporo-parietal craniotomy for subtotal resection of the tumor. The post-operative period was uneventful. Histopathology revealed a spindle cell tumor, which stained immunopositive for smooth muscle actin, vimentin, and S-100, yielding the diagnosis of LMS.
Conclusion:Primary intracranial LMS can rarely occur in immuno-competent adult patients and should be considered in the differential diagnosis of intraparenchymal lesions presenting with significant PTBE.
Keywords: Adult, immunocompetent, intracranial, leiomyosarcoma, primary
INTRODUCTION
Primary intracranial leiomyosarcomas (LMS) is a malignant neoplasm of smooth muscle cell origin[
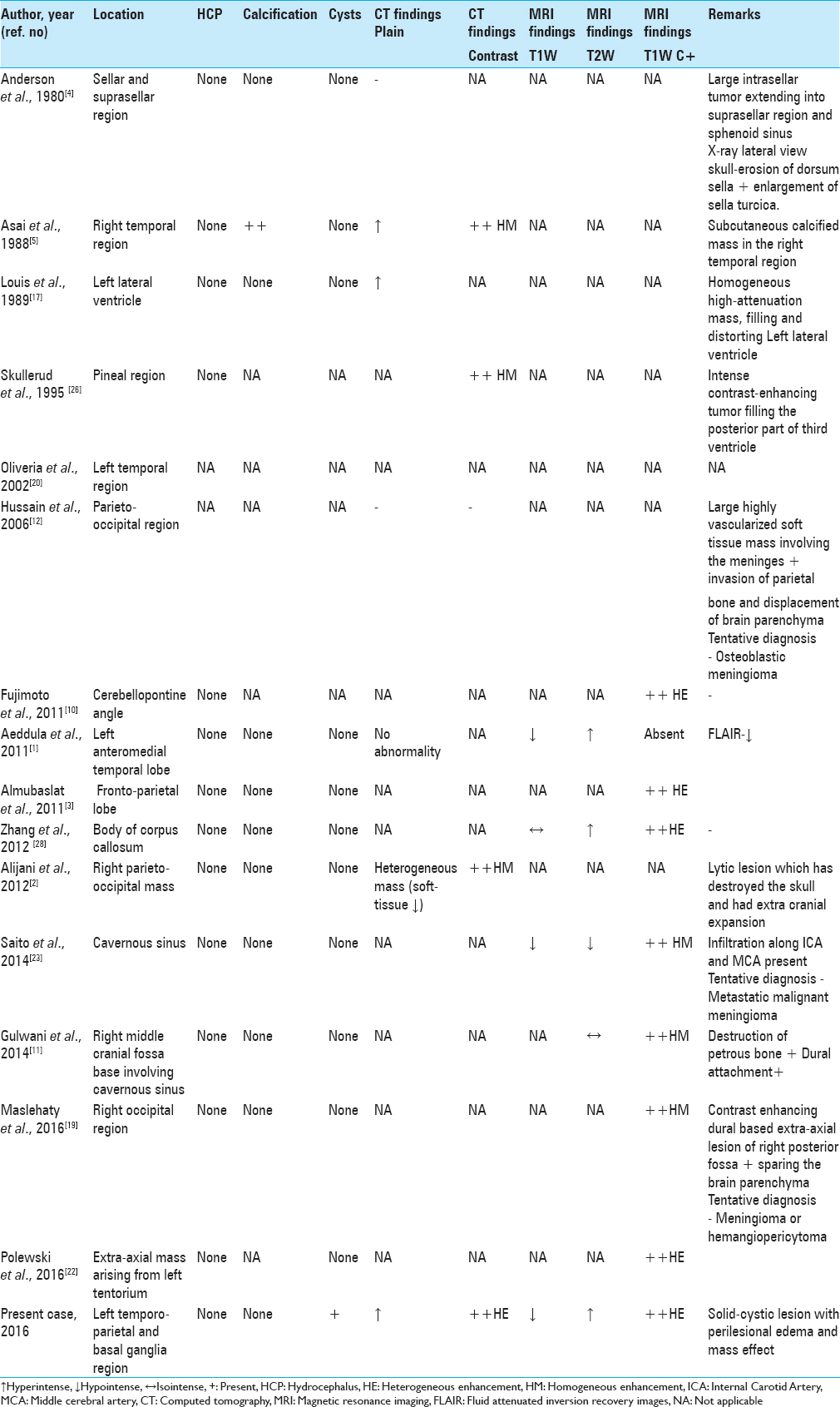
A thorough literature review revealed only 15 cases of primary intracranial LMSs in immuno-competent adult patients [
CASE REPORT
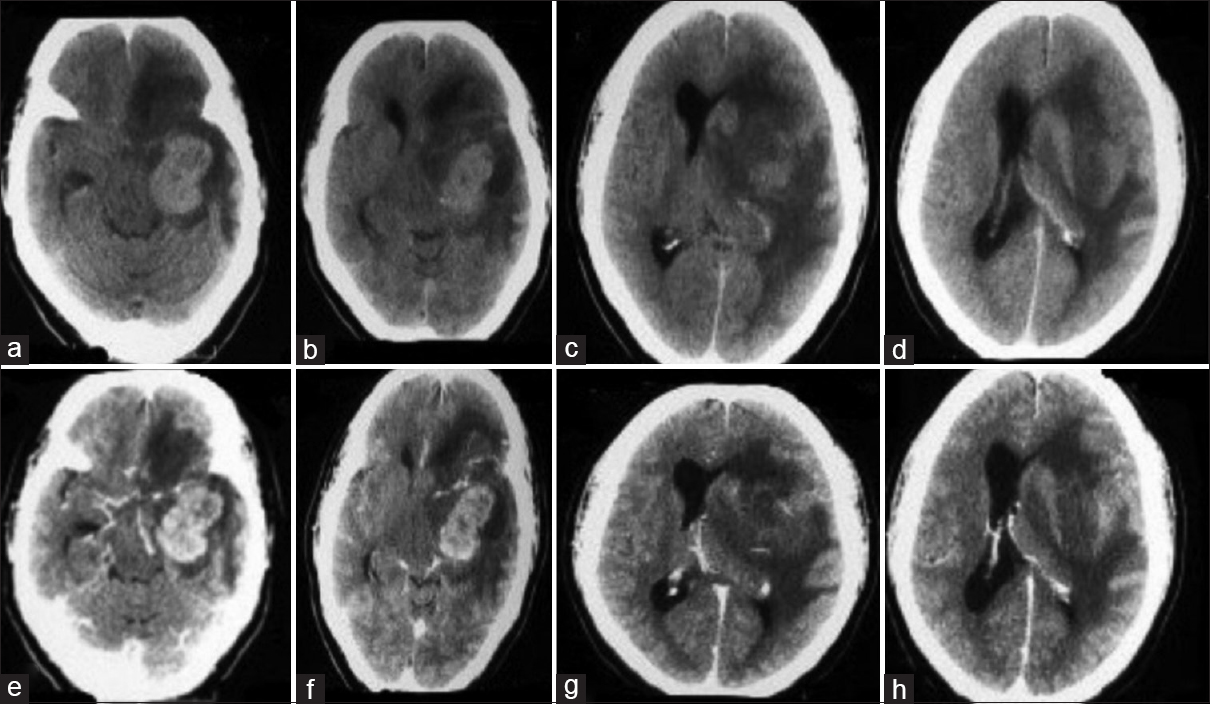
A 45-year-old man presented with a history of progressively increasing headaches, right-sided weakness, difficulties with speech for 4 months as well as a single generalized tonic clonic seizure. Clinical examination identified some papilledema, expressive aphasia, and right-sided hemiparesis (MRC grade 4/5). There was no history of intravenous drug use or radiation exposure. Routine laboratory studies returned as within normal limits and serology was negative for HIV, Hepatitis -B Virus (HBV), Hepatitis-C Virus (HCV), and Epstein Barr virus (EBV). No other comorbidities were noted. Head CT (native + contrast enhanced) demonstrated a heterogeneously enhancing lesion located in the left temporo-parietal areas with extension to the basal ganglia. Extensive perilesional edema and mass effect with midline shift towards the contralateral side was visible [
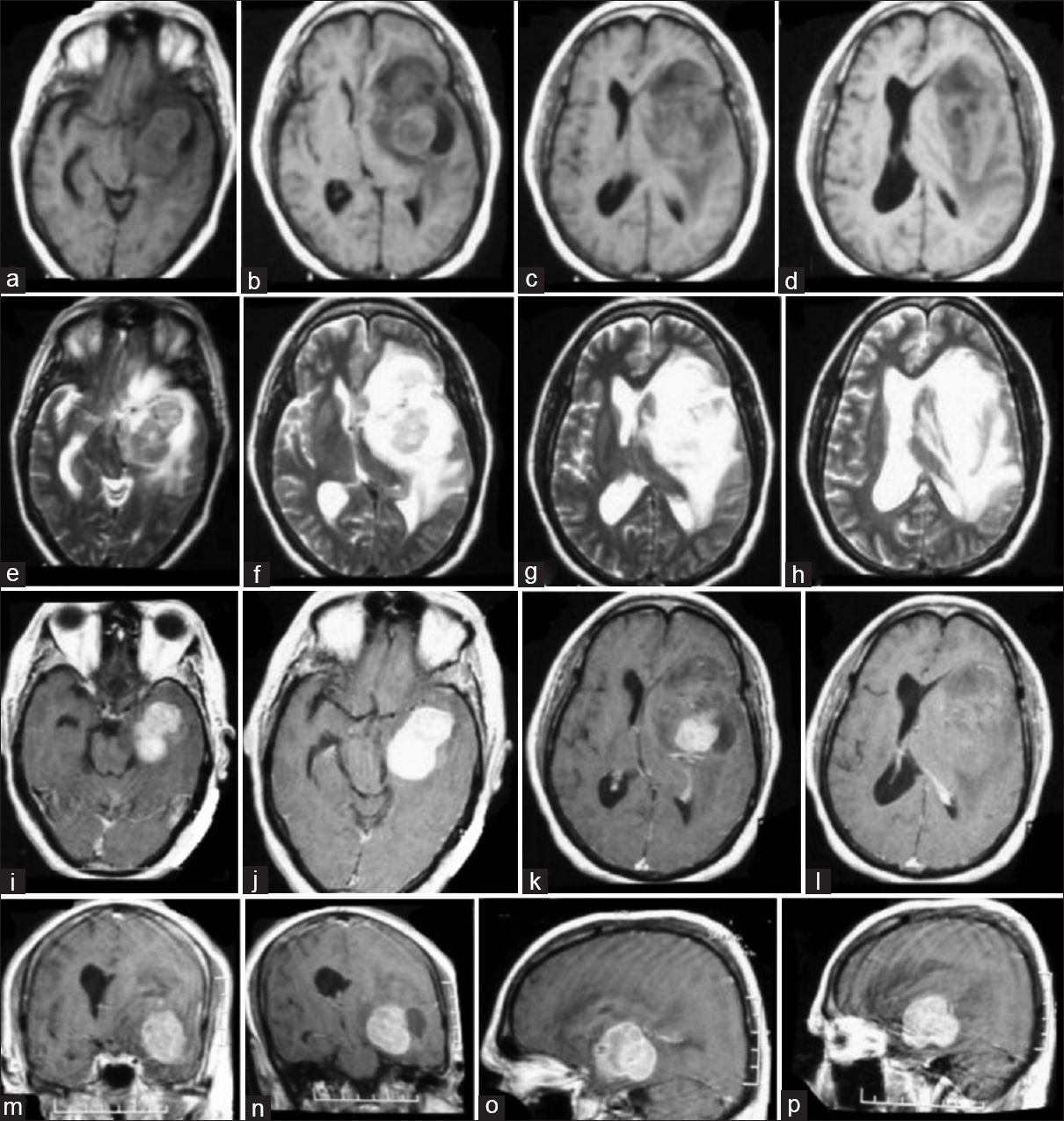
Brain MRI [
Figure 2
Magnetic resonance imaging (MRI) of tumor tissue showing a solid (predominant) and cystic lesion in the left frontotemporal and basal ganglia region with mass effect and midline shift with perilesional edema (a–d) T1W axial images; (e–h) T2W axial images; (i–l) T1 with gadolinium contrast axial images; (m, n) coronal contrast images; and (o, p) sagittal contrast images
Patient underwent a left temporo-parietal craniotomy and subtotal excision of the tumor, under image guidance. Intraoperatively, solid and cystic components (containing xanthochromic fluid) were encountered. The solid tumor component was greyish-white in appearance, showed firm consistency that required excision rather than removal by suction-irrigation. This part of the lesion was highly vascular leading to massive blood loss (approximately 2–2.5 liters). Since the tumor displayed poorly defined dissection planes only a subtotal excision could be achieved. Intraoperatively, he received 3 units of blood transfusion. The immediate post-operative period was uneventful and there was no deterioration in her neurological status. Post-operative CT scan showed some residual tumor without tumor cavity hematoma or bleed. Repeat CT scan 3 days after surgery showed resolving mid-line shift and edema. He received steroids in tapering doses for 7 days and was discharged home 10 days after surgery.
Pathology
Microscopic examination [
DISCUSSION
Primary intracranial LMS is an infrequent tumor of CNS arising from the mesenchymal cells of the cerebral blood vessels or duramater.[
Zhang et al.[
After a comprehensive literature review [
No specific symptoms have been identified that are associated with primary intracranial LMS, and symptoms vary largely based on tumor location.
It has been hypothesized that intraparenchymal LMSs originate from smooth muscle cells lining the intracranial blood vessels.[
Although the characteristic histopathological features of this rare entity have been highlighted and reviewed by other authors, there is a paucity of radiological data and description thereof in the literature.
Smith et al.[
Our purely intraparenchymal case and with solid and cystic components had massive perilesional edema and showed midline shift, both of which had never been reported in literature. The exact cause of peripherally located tumor cysts is not entirely known, although this may be due to degenerative changes, tumor cells secreting cystic fluid, or the formation of loculated fluid collections from scar tissue within or adjacent to the tumor.[
The profound peritumoral edema seen in our case may be due to the large tumor size, creating brain compression causing ischemia and secondary brain edema or could be due to enhanced vascularity, cellularity and mitotic activity of the tumor, since highly vascular tumors tend to have significant perilesional edema.
The diagnosis of LMS is based on demonstration of smooth muscle cells and their characteristic ultra- structural features on microscopy.[
Management
Since rather few case reports exist for this relatively rare clinical entity, there is no established standard treatment regimen for primary intracranial LMS. Based on our literature review, surgery remained the primary treatment, with the aim of obtaining negative surgical margins. Complete resection of the tumor was possible in majority of the reported cases, as shown in
There are also some reports[
The overall prognosis of LMS appears relatively poor and seems to dependent on tumor size and location as well as on the mitotic rate observed.[
Our recommendation is therefore to pursue aggressive multimodality treatment of this rare pathology until more extensive evidence is available about targeted treatment.
CONCLUSION
Primary intracranial LMS occurs very rarely in adult immune-competent patients, which makes the correct diagnosis in this setting challenging. Amongst lesions located purely intraparenchymally, LMS can rarely present with profound perilesional edema and mass effect as in our case, mimicking other malignant intracranial tumor entities. We hope that our literature review will contribute to a better understanding and increased awareness of this uncommon pathology.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Aeddula NR, Pathireddy S, Samaha T, Ukena T, Hosseinnezhad A. Primary intracranial leiomyosarcoma in an immunocompetent adult. J Clin Oncol. 2011. 29: e407-10
2. Aliana B, Yousef S, Armani A, Messiah A. Primary intracranial leiomyosarcoma. Arch Iran Med. 2013. 16: 606-7
3. Ambala M, Stone JC, Liu L, Xing Z. Primary intracranial leiomyosarcoma in an immunocompetent patient. Clin Neuropathology. 2011. 30: 154-7
4. Anderson WR, Cameron JD, Tsai SH. Primary intracranial leiomyosarcoma: Case report with ultrastructural study. J Neurosurgeon. 1980. 53: 401-5
5. Asia A, Yamada H, Murata S, Matsudo A, Satsuma K, Takemori T. Primary leiomyosarcoma of the dura mater: Case report. J Neuro Surge. 1988. 68: 308-11
6. Bartusch C, Afonso M, Piers-Luís AS, Gallagher A, Gemmaries M, Attunes L. Distant Metastases in Uterine Leiomyosarcomas: The Wide Variety of Body Sites and Time Intervals to Metastatic Relapse. Int J Gynecol Pathol. 2016. p.
7. Brown HG, Burger PC, Olivi A, Sills AK, Barditch-Crovo PA, Lee RR. Intracranial leiomyosarcoma in a patient with AIDS. Neuroradiology. 1999. 41: 35-9
8. Eckhardt BP, Brandner S, Zollikofer CL, Wentz KU. Primary cerebral leiomyosarcoma in a child. Pediatr Radiol. 2004. 34: 495-8
9. Flannery T, Kano H, Niranjan A, Monaco EA, Flickinger JC, Kofler J. Gamma knife radiosurgery as a therapeutic strategy for intracranial sarcomatous metastases. Int J Radiat Oncol Biol Phys. 2010. 76: 513-9
10. Fujimoto Y, Hirato J, Wakayama A, Yoshimine T. Primary intracranial leiomyosarcoma in an immunocompetent patient: Case report. J Neurooncol. 2011. 103: 785-90
11. Gulwani HV, Garg N. Primary extradural leiomyosarcoma involving cavernous sinus in an immunocompetent patient. Indian J Neurosurg. 2014. 3: 115-7
12. Hussain S, Nanda A, Fowler M, Ampil FL, Burton GV. Primary intracranial leiomyosarcoma: Report of a case and review of the literature. Sarcoma. 2006. 2006: 52140-
13. Jhas S, Henriques L, Hawkins C, Bouffet E, Rutka JT. An intracranial leiomyosarcoma in a child with neurofibromatosis type 1. Can J Neurol Sci. 2009. 36: 491-5
14. Lee KJ, Joo WI, Rha HK, Park HK, Chough JK, Hong YK. Peritumoral brain edema in meningiomas: Correlations between magnetic resonance imaging, angiography, and pathology. Surg Neurol. 2008. 69: 350-5
15. Lee TT, Page LK. Primary cerebral leiomyosarcoma. Clin Neurol Neurosurg. 1997. 99: 210-2
16. Li NY. Primary leiomyosarcoma of the pineal gland: A case report [in Chinese]. Zhonghua Zhong Liu Za Zhi. 1987. 9: 463-4
17. Louis DN, Richardson EP, Dickersin GR, Petrucci DA, Rosenburg AE, Ojemann RG. Primary intra cranial leiomyosarcoma: Case report. J Neurosurg. 1989. 71: 279-82
18. Maslehaty H, Nabavi A, Mehdorn M. Primary intracranial leiomyosarcoma- case report and principles for treatment. J Neurol Neuromed. 2016. 1: 16-20
19. Mathieson CS, St George EJ, Stewart W, Sastry J, Jamal S. Primary intracranial leiomyosarcoma: A case report and review of the literature. Childs Nerv Syst. 2009. 25: 1013-7
20. Oliveira AM, Scheithauer BW, Salomao DR, Parisi JE, Burger PC, Nascimento AG. Primary sarcomas of the brain and spinal cord: A study of 18 cases. Am J Surg Pathol. 2002. 26: 1056-63
21. Paulus W, Slowik F, Jellinger K. Primary intracranial sarcomas: Histopathological features of 19 cases. Histopathology. 1991. 18: 395-402
22. Polewski PJ, Smith AL, Conway PD, Marinier DE. Primary CNS Leiomyosarcoma in an Immunocompetent Patient. J Oncol Pract. 2016. 12: 827-9
23. Saito N, Akoi K, Hirari N, Ishii M, Fujita S, Hiramoto Y. Primary intracranial leiomyosarcoma of the cavernous sinus: Case Report. Austin J Med Oncol. 2014. 1: 2-
24. Shepard MJ, Fezeu F, Lee CC, Sheehan JP. Gamma knife radiosurgery for the treatment of gynecologic malignancies metastasizing to the brain: Clinical article. J Neurooncol. 2014. 120: 515-22
25. Sivendran S, Vidal CI, Barginear MF. Primary intracranial leiomyosarcoma in an HIV-infected patient. Int J Clin Oncol. 2011. 16: 63-6
26. Skullerud K, Stenwig AE, Brandtzaeg P, Nesland JM, Kerty E, Langmoen I. Intracranial primary leiomyosarcoma arising in a teratoma of the pineal area. Clin Neuropathol. 1995. 14: 245-8
27. Smith AB, Horkanyne-Szakaly I, Schroeder JW, Rushing EJ. From the radiologic pathology archives: Mass lesions of the dura: Beyond meningioma-radiologic-pathologic correlation. Radiographics. 2014. 34: 295-312
28. Zhang H, Dong L, Huang Y, Zhang B, Ma H, Zhou Y. Primary intracranial leiomyosarcoma: Review of the literature and presentation of a case. Onkologie. 2012. 35: 609-16