- Department of Neurosurgery, University of Rochester Medical Center, Rochester, New York, United States
Correspondence Address:
Keaton F. Piper
Department of Neurosurgery, University of Rochester Medical Center, Rochester, New York, United States
DOI:10.4103/sni.sni_306_17
Copyright: © 2017 Surgical Neurology International This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Keaton F. Piper, Samuel B. Tomlinson, Gabrielle Santangelo, Joseph Van Galen, Ian DeAndrea-Lazarus, James Towner, Kristopher T. Kimmell, Howard Silberstein, George Edward Vates. Risk factors for wound complications following spine surgery. 01-Nov-2017;8:269
How to cite this URL: Keaton F. Piper, Samuel B. Tomlinson, Gabrielle Santangelo, Joseph Van Galen, Ian DeAndrea-Lazarus, James Towner, Kristopher T. Kimmell, Howard Silberstein, George Edward Vates. Risk factors for wound complications following spine surgery. 01-Nov-2017;8:269. Available from: http://surgicalneurologyint.com/surgicalint-articles/risk-factors-for-wound-complications-following-spine-surgery/
Abstract
Background:Wound complications, including surgical site infections (SSIs) and wound dehiscence, are among the most common complications following spine surgery often leading to readmission. The authors sought to identify preoperative characteristics predictive of wound complications after spine surgery.
Methods:The American College of Surgeons National Surgical Quality Improvement Program database for years 2012–2014 was reviewed for patients undergoing spine surgery, defined by the Current Procedural Terminology codes. Forty-four preoperative and surgical characteristics were analyzed for associations with wound complications.
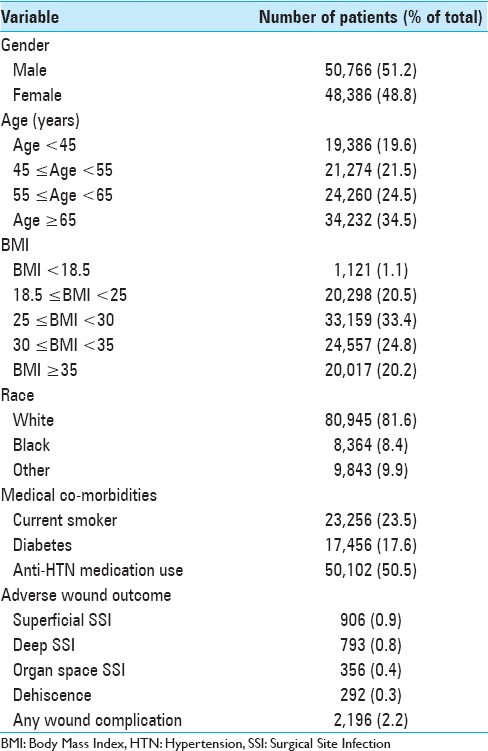
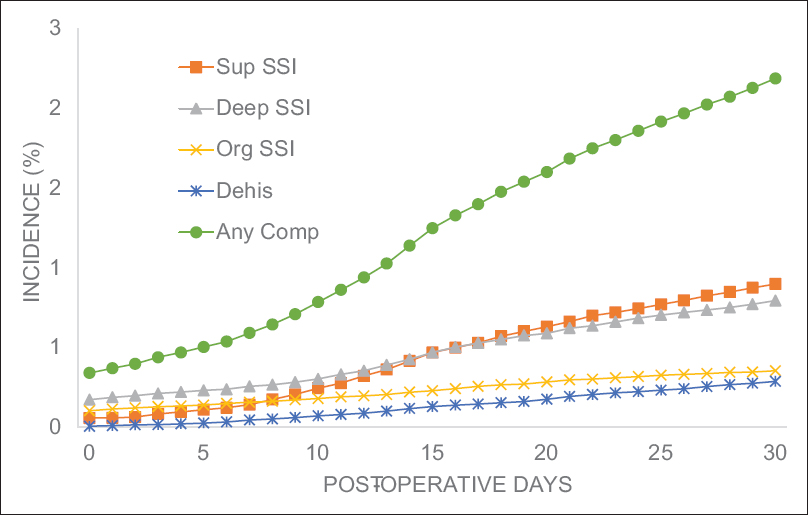
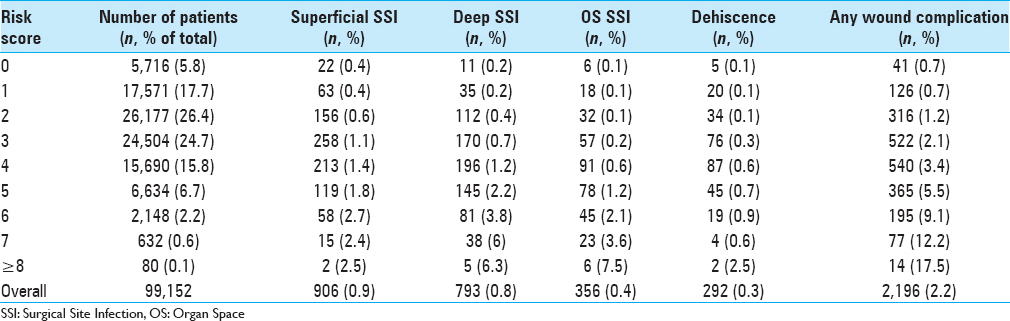
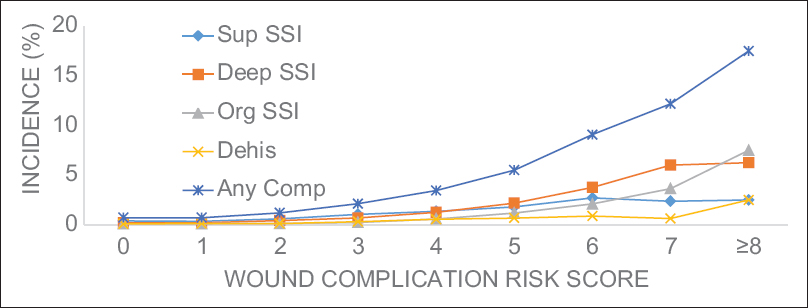
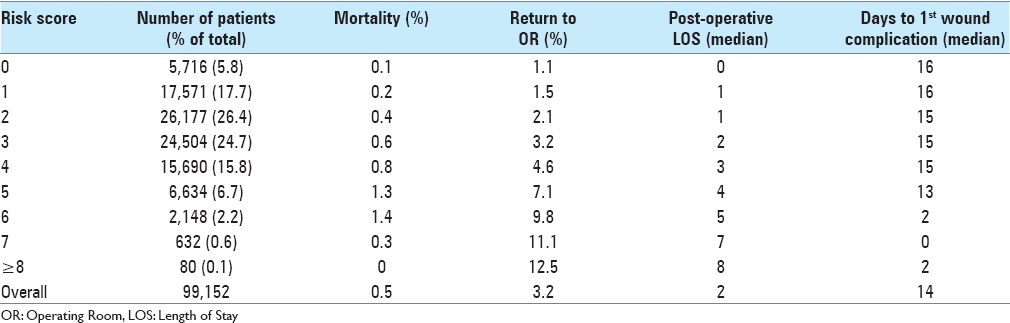
Results:Of the 99,152 patients included in this study, 2.2% experienced at least one wound complication (superficial SSI: 0.9%, deep SSI: 0.8%, organ space SSI: 0.4%, and dehiscence: 0.3%). Multivariate binary logistic regression testing found 10 preoperative characteristics associated with wound complications: body mass index ≥30, smoker, female, chronic steroid use, hematocrit 3 hours. A risk score for each patient was created from the number of characteristics present. Receiver operating characteristic curves of the unweighted and weighted risk scores generated areas under the curve of 0.701 (95% CI: 0.690–0.713) and 0.715 (95% CI: 0.704–0.726), respectively. Patients with unweighted risk scores >7 were 25-fold more likely to develop a wound complication compared to patients with scores of 0. In addition, mortality rate, reoperation rate, and total length of stay each increased nearly 10-fold with increasing risk score.
Conclusion:This study introduces a novel risk score for the development of wound dehiscence and SSIs in patients undergoing spine surgery, using new risk factors identified here.
Keywords: Spine surgery, surgical site infection, wound dehiscence, wound complication, risk factors
INTRODUCTION
The increasing cost associated with spine surgery is a well-known problem affecting the United States (US) healthcare system; this is becoming more important with the increasing prevalence of spine surgeries. From 2002 to 2007, the rate of complex spine surgery increased almost 15-fold in the Medicare population.[
Among the most common postoperative complications associated with readmissions are wound complications, including surgical site infections (SSIs) and dehiscence.[
Given the relatively low percentage of wound infections and dehiscence in spine surgery, it is difficult to develop a cost-effective intervention for reducing these rates. One approach to this problem is to identify patients who are at an increased risk of wound complications and may benefit from more intensive preventative wound care. There have been various effective interventions aimed at reducing wound complications.[
In the present study, we sought to determine preoperative characteristics independently associated with wound complications in patients undergoing spine surgery. Using these factors, we developed a novel risk score for this cohort that may be used to calculate a patient's risk of developing a wound complication including organ-space SSI, deep incisional SSI, superficial SSI, or dehiscence. Further validation of this methodology may allow clinicians to anticipate high-risk cases and adjust perioperative management with the goal of reducing occurrences of wound complications.
MATERIALS AND METHODS
Data acquisition
Data were collected from the National Surgical Quality Improvement Project (NSQIP) dataset during the years 2012–2014. Organized by the American College of Surgeons (ACS), the NSQIP database is a collection of perioperative data sourced from deidentified surgical cases at over 700 hospitals in the US. Data allocation at each participating site is performed by an ACS-trained Surgical Clinician Reviewer (SCR), who collects information in a standardized manner, maintains a degree of separation from the hospital's physicians, and undergoes regular audits.[
Univariate and multivariate analyses
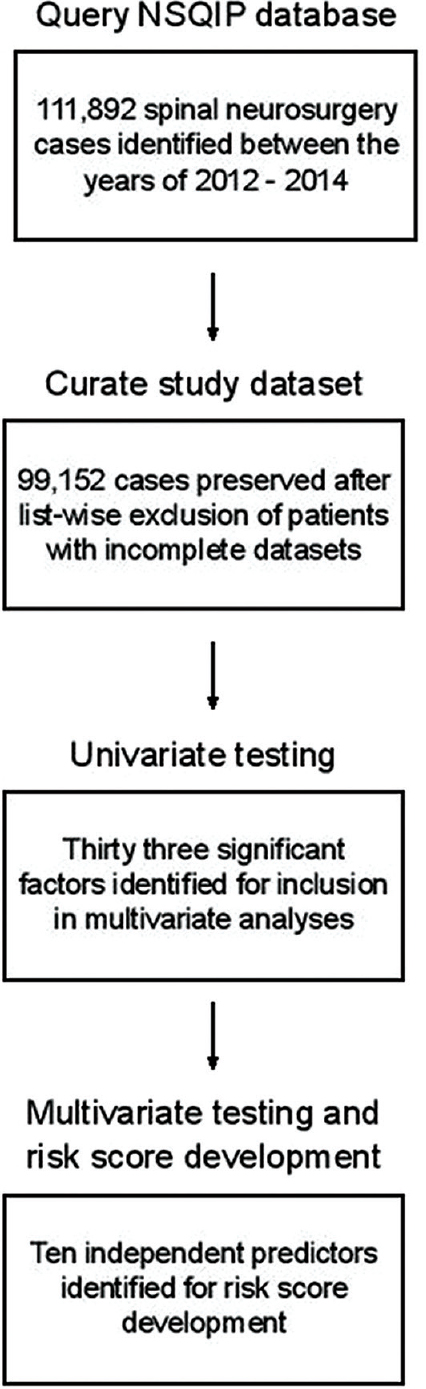
All patients who underwent spine surgery between 2012 and 2014 were identified by the Current Procedural Terminology (CPT) codes [
Four specific wound complications tracked by NSQIP were examined in this study: dehiscence, superficial SSI, deep SSI, and organ-space SSI. To construct a generalizable risk score, a composite binary outcome representing the occurrence of any wound complication was defined. Preoperative characteristics underwent univariate analysis for association with the composite wound complication outcome measure using Chi-square tests and Fischer's exact tests where appropriate. Variables were gated for entry into multivariate modeling using a P value threshold of P = 0.01. These factors were then submitted into a multivariate binary logistical regression model for association with the “any wound complication” composite outcome (entry level = 0.01, exit = 0.05). Statistical significance was determined by using an adjusted α from a Holm-Bonferroni correction. For each factor, odds ratios were calculated. Statistical analysis was performed with a combination of Statistical Analysis Software (SAS Institute Inc., Cary, NC) and Statistical Package for the Social Science (SPSS) software (version 24.0 IBM).
Risk score computation
Ten preoperative characteristics deemed statistically significant by multivariate analysis were used in generating weighted and unweighted risk scores. For the unweighted risk score, each independent risk factor was given a value of 1 when present. The factors were summed to create each individual patient's risk score, ranging from 0 to 10. A weighted risk score was created using adjusted multivariate odds ratios, consistent with previous risk score computations.[
RESULTS
In total, 99,152 spine surgery cases with complete datasets were analyzed [
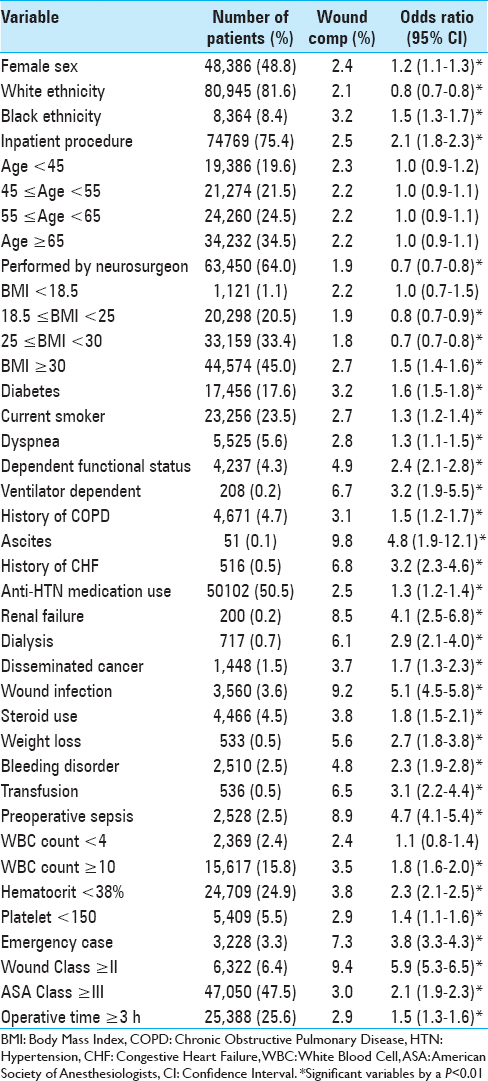
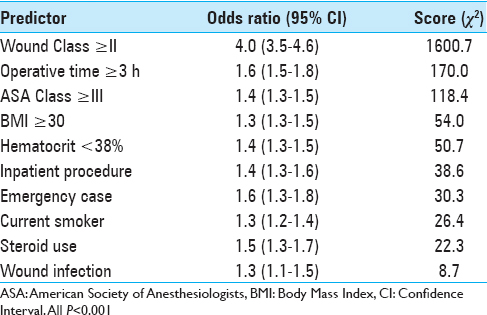
Univariate analysis identified 33 characteristics significantly related to an increased risk of developing a postoperative wound complication [
DISCUSSION
In this study, we examined preoperative factors associated with postoperative wound complications following spine surgery. Using a sample encompassing nearly 100,000 cases from a national surgical database, we characterized wound complication rates and identified independent predictors associated with the development of at least one wound complication. Further, we implemented a novel scoring system for stratifying preoperative risk and demonstrated its performance among our sample. This study provides the largest to-date analysis of SSIs and dehiscence in spine surgery. Patient characteristics and wound complication rates [
Several of the characteristics have previously been reported as potential risk factors for SSI or dehiscence in specific spine surgeries. For example, a recent study of posterior cervical spine surgery identified BMI >35 kg/m2, chronic steroid use, prolonged operation time, hematocrit <33%, and ASA class >2 as independent risk factors for postoperative SSI.[
Our study is the first to identify risk factors for all wound complications including dehiscence and SSIs in patients undergoing spine surgery. For wound complications, specific factors such as inpatient status and emergent case classification are first reported here. Although some risk factors associated with dehiscence have been published previously in relation to other types of surgery, they have not been associated with wound complications specifically after spine surgery.[
Existing NSQIP-derived risk scores have shown promise in predicting outcomes within other surgical fields.[
Although NSQIP-based risk scores have been introduced within many different specialties,[
One notable advance of the present study is the rigorous vetting of preoperative data to exclude patients with incomplete datasets and missing variables. The handling of missing data in NSQIP-based studies has come under increased scrutiny and excluding patients with incomplete data entry is expected to address these concerns.[
Future work will be aimed at extending this risk score to include spine-specific variables and outcomes beyond 30 days for a more comprehensive scoring system. Additionally, further validation of this risk score is important by measuring the clinical response of providing preventative interventions for wound complications among identified “high-risk” patients in attempt to implement targeted cost-effective interventions. Such interventions include systemic antibiotics, local intraoperative antibiotics, multimodal preoperative skin preparation, negative pressure wound therapy, more extensive incisional closure (e.g. muscle flap closure), and more extensive postoperative wound care.[
CONCLUSION
This study introduces a novel preoperative risk score for the development of wound dehiscence and SSIs in patients undergoing spinal surgery. The results suggest that a subset of spine surgery patients account for a disproportionate percentage of adverse wound outcomes, suggesting that high-risk patients may be identified before surgery. Further development of this risk score may prove useful for identifying high-risk patients that might benefit from more intensive wound management.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Adogwa O, Fatemi P, Perez E, Moreno J, Gazcon GC, Gokaslan ZL. Negative pressure wound therapy reduces incidence of postoperative wound infection and dehiscence after long-segment thoracolumbar spinal fusion: A single institutional experience. Spine J. 2014. 14: 2911-7
2. Akins PT, Harris J, Alvarez JL, Chen Y, Paxton EW, Bernbeck J. Risk Factors Associated With 30-day Readmissions After Instrumented Spine Surgery in 14,939 Patients: 30-day readmissions after instrumented spine surgery. Spine (Phila Pa 1976). 2015. 40: 1022-32
3. Aksamija G, Mulabdic A, Rasic I, Aksamija L. Evaluation of Risk Factors of Surgical Wound Dehiscence in Adults After Laparotomy. Med Arch. 2016. 70: 369-72
4. Alan N, Seicean A, Seicean S, Neuhauser D, Weil RJ. Impact of preoperative anemia on outcomes in patients undergoing elective cranial surgery. J Neurosurg. 2014. 120: 764-72
5. Althumairi AA, Canner JK, Gearhart SL, Safar B, Fang SH, Wick EC. Risk factors for wound complications after abdominoperineal excision: Analysis of the ACS NSQIP database. Color Dis. 2016. 18: O260-6
6. Andrew Glennie R, Dea N, Street JT. Dressings and drains in posterior spine surgery and their effect on wound complications. J Clin Neurosci. 2015. 22: 1081-7
7. Bekelis K, Desai A, Bakhoum SF, Missios S. A predictive model of complications after spine surgery: The National Surgical Quality Improvement Program (NSQIP) 2005-2010. Spine J. 2014. 14: 1247-55
8. Dasenbrock HH, Devine CA, Liu KX, Gormley WB, Claus EB, Smith TR. Thrombocytopenia and craniotomy for tumor: A National Surgical Quality Improvement Program analysis. Cancer. 2016. 122: 1708-17
9. Dennis HH, Wei DT, Darren KZ, Shantakumar JT, Kumar N, Lau LL. Is Intraoperative Local Vancomycin Powder the Answer to Surgical Site Infections in Spine Surgery?. Spine (Phila Pa 1976). 2016. p.
10. Deyo RA, Mirza SK, Martin BI, Kreuter W, Goodman DC, Jarvik JG. Trends, major medical complications, and charges associated with surgery for lumbar spinal stenosis in older adults. JAMA. 2010. 303: 1259-65
11. Dumanian GA, Ondra SL, Liu J, Schafer MF, Chao JD. Muscle flap salvage of spine wounds with soft tissue defects or infection. Spine (Phila Pa 1976). 2003. 28: 1203-11
12. Featherall J, Miller JA, Bennett EE, Lubelski D, Wang H, Khalaf T. Implementation of an Infection Prevention Bundle to Reduce Surgical Site Infections and Cost Following Spine Surgery. JAMA Surg. 2016. 151: 988-90
13. Fink AS, Campbell DA, Mentzer RM, Henderson WG, Daley J, Bannister J. The National Surgical Quality Improvement Program in non-veterans administration hospitals: Initial demonstration of feasibility. Ann Surg. 2002. 236: 344-
14. Gani F, Canner JK, Pawlik TM. Use of the Modified Frailty Index in the American College of Surgeons National Surgical Improvement Program Database: Highlighting the Problem of Missing Data. JAMA Surg. 2017. 152: 205-7
15. Giannopoulos G, Raisakis K, Synetos A, Davlouros P, Hahalis G, Alexopoulos D. A predictive score of radial artery spasm in patients undergoing transradial percutaneous coronary intervention. Int J Cardiol. 2015. 188: 76-80
16. Goz V, Weinreb JH, McCarthy I, Schwab F, Lafage V, Errico TJ. Perioperative complications and mortality after spinal fusions: Analysis of trends and risk factors. Spine (Phila Pa 1976). 2013. 38: 1970-6
17. Guillamondegui OD, Gunter OL, Hines L, Martin BJ, Gibson W, Clarke PC. Using the National Surgical Quality Improvement Program and the Tennessee Surgical Quality Collaborative to improve surgical outcomes. J Am Coll Surg. 2012. 214: 706-9
18. Hall BL, Hamilton BH, Richards K, Bilimoria KY, Cohen ME, Ko CY. Does surgical quality improve in the American College of Surgeons National Surgical Quality Improvement Program: An evaluation of all participating hospitals. Ann Surg. 2009. 250: 363-76
19. Jalai CM, Worley N, Poorman GW, Cruz DL, Vira S, Passias PG. Surgical site infections following operative management of cervical spondylotic myelopathy: Prevalence, predictors of occurence, and influence on peri-operative outcomes. Eur Spine J. 2016. 25: 1891-6
20. Khuri SF, Daley J, Henderson W, Hur K, Demakis J, Aust JB. The Department of Veterans Affairs’ NSQIP: The first national, validated, outcome-based, risk-adjusted, and peer-controlled program for the measurement and enhancement of the quality of surgical care. National VA Surgical Quality Improvement Program. Ann Surg. 1998. 228: 491-507
21. Kimmell KT, Algattas H, Joynt P, Schmidt T, Jahromi BS, Silberstein HJ. Risk Modeling Predicts Complication Rates for Spinal Surgery. Spine (Phila Pa 1976). 2015. 40: 1836-41
22. Kuhns BD, Lubelski D, Alvin MD, Taub JS, McGirt MJ, Benzel EC. Cost and quality of life outcome analysis of postoperative infections after subaxial dorsal cervical fusions. J Neurosurg Spine. 2015. 22: 381-6
23. De la Garza-Ramos R, Abt NB, Kerezoudis P, McCutcheon BA, Bydon A, Gokaslan Z. Deep-wound and organ-space infection after surgery for degenerative spine disease: An analysis from 2006 to 2012. Neurol Res. 2016. 38: 117-23
24. Labler L, Keel M, Trentz O, Heinzelmann M. Wound conditioning by vacuum assisted closure (V.A.C) in postoperative infections after dorsal spine surgery. Eur Spine J. 2006. 15: 1388-96
25. Lau D, Chan AK, Theologis AA, Chou D, Mummaneni P V, Burch S. Costs and readmission rates for the resection of primary and metastatic spinal tumors: A comparative analysis of 181 patients. J Neurosurg Spine. 2016. 25: 366-78
26. Lee MJ, Cizik AM, Hamilton D, Chapman JR. Predicting medical complications after spine surgery: A validated model using a prospective surgical registry. Spine J. 2014. 14: 291-9
27. Lewkonia P, DiPaola C, Street J. Incidence and risk of delayed surgical site infection following instrumented lumbar spine fusion. J Clin Neurosci. 2016. 23: 76-80
28. McCormack RA, Hunter T, Ramos N, Michels R, Hutzler L, Bosco JA. An analysis of causes of readmission after spine surgery. Spine (Phila Pa 1976). 2012. 37: 1260-6
29. Meng F, Cao J, Meng X. Risk factors for surgical site infections following spinal surgery. J Clin Neurosci. 2015. 22: 1862-6
30. Piper K, Algattas H, DeAndrea-Lazarus IA, Kimmell KT, Li YM, Walter KA. Risk factors associated with venous thromboembolism in patients undergoing spine surgery. J Neurosurg Spine. 2017. p. 26-
31. Pull ter Gunne AF, Cohen DB. Incidence, prevalence, and analysis of risk factors for surgical site infection following adult spinal surgery. Spine (Phila Pa 1976). 2009. 34: 1422-8
32. Radcliff KE, Neusner AD, Millhouse PW, Harrop JD, Kepler CK, Rasouli MR. What is new in the diagnosis and prevention of spine surgical site infections. Spine J. 2015. 15: 336-47
33. Saleh A, Thirukumaran C, Mesfin A, Molinari RW. Complications and readmission after lumbar spine surgery in elderly patients: An analysis of 2,320 patients. Spine J. 2017. p.
34. Sandy-Hodgetts K, Carville K, Leslie GD. Determining risk factors for surgical wound dehiscence: A literature review. Int Wound J. 2015. 12: 265-75
35. Schimmel JJ, Horsting PP, de Kleuver M, Wonders G, van Limbeek J. Risk factors for deep surgical site infections after spinal fusion. Eur Spine J. 2010. 19: 1711-9
36. Schweizer ML, Cullen JJ, Perencevich EN, Vaughan Sarrazin MS. Costs Associated With Surgical Site Infections in Veterans Affairs Hospitals. JAMA Surg. 2014. 149: 575-81
37. Sebastian AS, Polites SF, Glasgow AE, Habermann EB, Cima RR, Kakar S. Current Quality Measurement Tools Are Insufficient to Assess Complications in Orthopedic Surgery. J Hand Surg Am. 2017. 42: 10-15.e1
38. Sielatycki JA, Parker SL, Godil SS, McGirt MJ, Devin CJ. Do Patient Demographics and Patient-Reported Outcomes Predict 12-Month Loss to Follow-Up After Spine Surgery?. Spine (Phila Pa 1976). 2015. 40: 1934-40
39. Wachter D, Bruckel A, Stein M, Oertel MF, Christophis P, Boker DK. 2-Octyl-cyanoacrylate for wound closure in cervical and lumbar spinal surgery. Neurosurg Rev. 2010. 33: 483-9
40. van Walraven C, Musselman R. The Surgical Site Infection Risk Score (SSIRS): A Model to Predict the Risk of Surgical Site Infections. PLoS One. 2013. 8: e67167-
41. Wang T, Wang H, Yang DL, Jiang LQ, Zhang LJ, Ding WY. Factors predicting surgical site infection after posterior lumbar surgery: A multicenter retrospective study. Med. 2017. 96: e6042-
42. Webb ML, Nelson SJ, Save A, Cui J, Lukasiewicz AM, Samuel AM. Of 20,376 Lumbar Discectomies, 2.6% of Patients Readmitted within 30 Days: Surgical Site Infection, Pain, and Thromboembolic Events are the Most Common Reasons for Readmission. Spine (Phila Pa 1976). 2016. p.
43. Yeramaneni S, Robinson C, Hostin R. Impact of spine surgery complications on costs associated with management of adult spinal deformity. Curr Rev Musculoskelet Med. 2016. 9: 327-32