- Department of Neurosurgery, Karolinska University Hospital, Stockholm, Sweden
- Department of Neuroradiology, Karolinska University Hospital, Stockholm, Sweden
- Department of Clinical Pathology, Karolinska University Hospital, Stockholm, Sweden
- Department of Neurophysiology, Karolinska University Hospital, Stockholm, Sweden
- Department of Clinical Neuroscience, Karolinska Institutet, Stockholm, Sweden
- Department of Neurosurgery, Copenhagen University Hospital Rigshospitalet, Copenhagen, Denmark
Correspondence Address:
Alia Shamikh
Department of Neurosurgery, Karolinska University Hospital, Stockholm, Sweden
DOI:10.4103/sni.sni_482_16
Copyright: © 2017 Surgical Neurology International This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Georges Sinclair, Heather Martin, Alia Shamikh, Amir Samadi, Gerald Cooray, Jiri Bartek, Yehya Al-Saffar, Mikael Svensson, Ernest Dodoo. Salvage gamma knife radiosurgery in the management of dysembryoplastic neuroepithelial tumors: Long-term outcome in a single-institution case series. 09-Aug-2017;8:174
How to cite this URL: Georges Sinclair, Heather Martin, Alia Shamikh, Amir Samadi, Gerald Cooray, Jiri Bartek, Yehya Al-Saffar, Mikael Svensson, Ernest Dodoo. Salvage gamma knife radiosurgery in the management of dysembryoplastic neuroepithelial tumors: Long-term outcome in a single-institution case series. 09-Aug-2017;8:174. Available from: http://surgicalneurologyint.com/surgicalint-articles/salvage-gamma-knife-radiosurgery-in-the-management-of-dysembryoplastic-neuroepithelial-tumors-long%e2%80%91term-outcome-in-a-single%e2%80%91institution-case-series/
Abstract
Background:Dysembryoplastic neuroepithelial tumors (DNT/DNET) are rare epileptogenic tumors. Microsurgery remains the best treatment option, although case reports exist on the use of gamma knife radiosurgery (GKRS) in selected cases. We investigated the long-term outcome of GKRS-treated DNTs at our institution in the context of current diagnostic and treatment options.
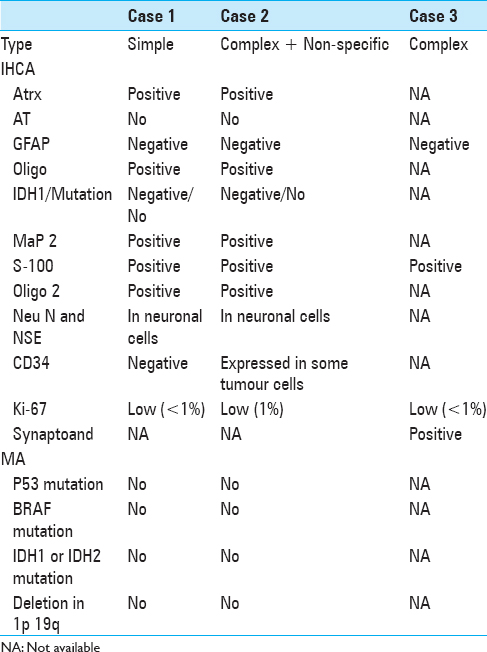
Case Descriptions:We conducted a retrospective review of three consecutive adult patients (≥18 years) treated with salvage GKRS between 2002 and 2010 at Karolinska University Hospital, Stockholm, Sweden. The case series was supplemented by a review of current literature. A 20-year-old male underwent subtotal resection (STR) in 1997 and 2002 of DNT resulting in temporary control of intractable epilepsy despite antiepileptic drug treatment (AED). Long-term seizure control was obtained after GKRS of two separate residual DNT components along the surgical margin (2005 and 2010). A 27-year-old male undergoing gross total resection of the contrast-enhancing portion of a DNT (1999) resulted in temporary control of intractable epilepsy despite AEDs; lasting clinical control of seizures was achieved in 2002 after GKRS of a small, recurrent DNT component. A 28-year-old male underwent STR of DNT (1994 and 2004) resulting in temporary control of intractable epilepsy. Lasting seizure control was gained after GKRS of a residual tumor (2005).
Conclusion:GKRS as performed in our series was effective in terms of tumor and seizure control. No adverse radiation effects were recorded. Prospective studies are warranted to establish the role of GKRS in the treatment of DNTs.
Keywords: Dysembryoplastic neuroepithelial tumors, ENGEL score, gamma knife radiosurgery, gross total resection, intractable epilepsy, subtotal resection
INTRODUCTION
Dysembryoplastic neuroepithelial tumors (DNTs or DNETs) were first reported by Daumas–Duport et al. in 1988.[
MATERIALS AND METHODS
With institutional approval, we conducted a retrospective review of three consecutive adult patients (≥18 years) treated with GKRS as salvage therapy between 2002 and 2010 at Karolinska University Hospital, Stockholm, Sweden. The data were collected from hospital medical records and our Leksell Gamma Plan system. The case series was supplemented by a review of the current literature. Reviewing the current literature published on PubMed, only two case reports on GKRS treating DNTs were found.[
CASE SUMMARIES
Case 1
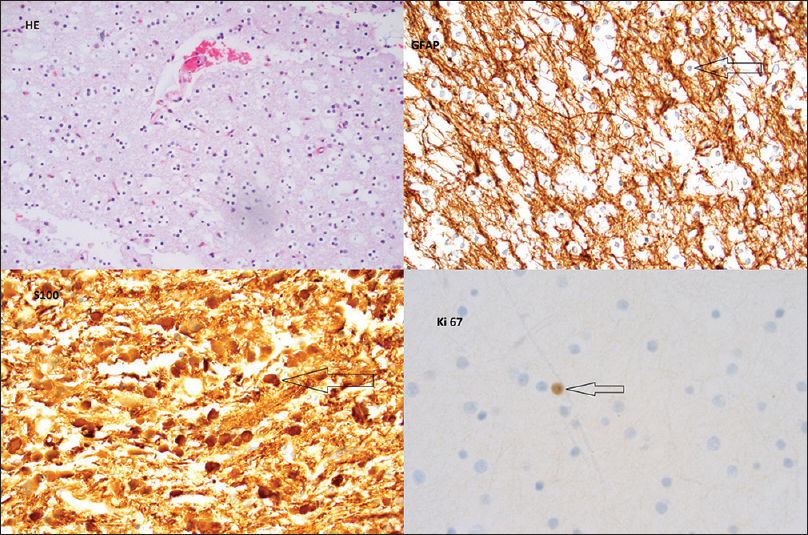
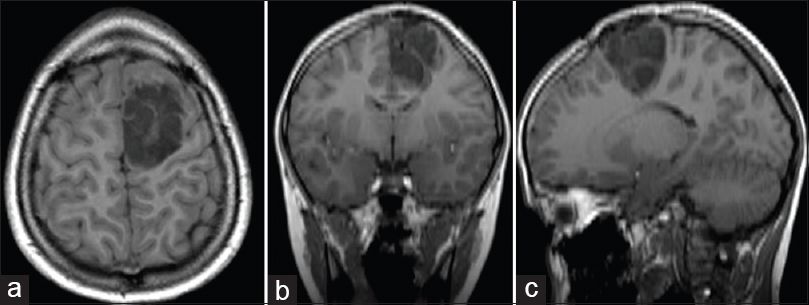
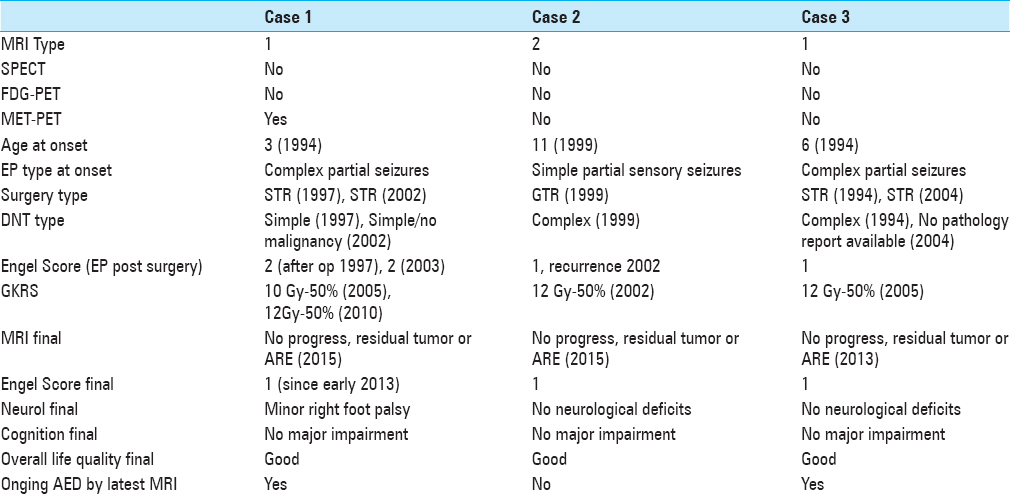
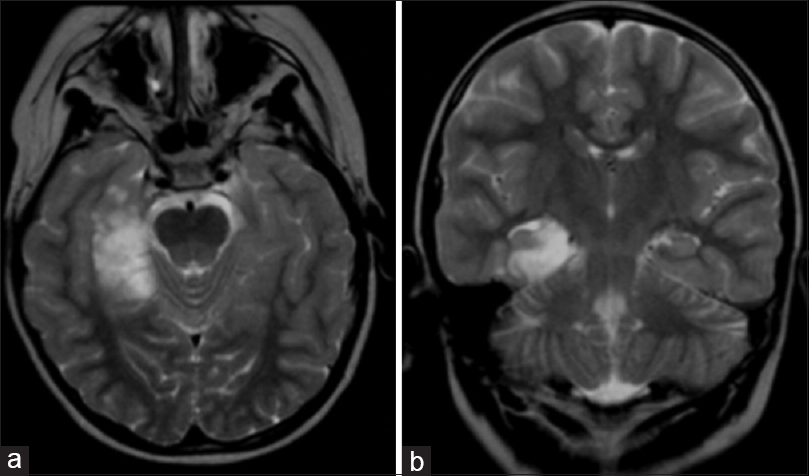
A 20-year old male developed complex partial seizures by the age of 3 (April 1996). Magnetic resonance imaging (MRI) revealed a nonenhancing T2/FLAIR hyperintense, well-defined mass in the left frontal lobe. After a subtotal resection (STR) in 1997, histopathological analysis concluded that the tumor was a DNT of so-called simple form [
Figure 1
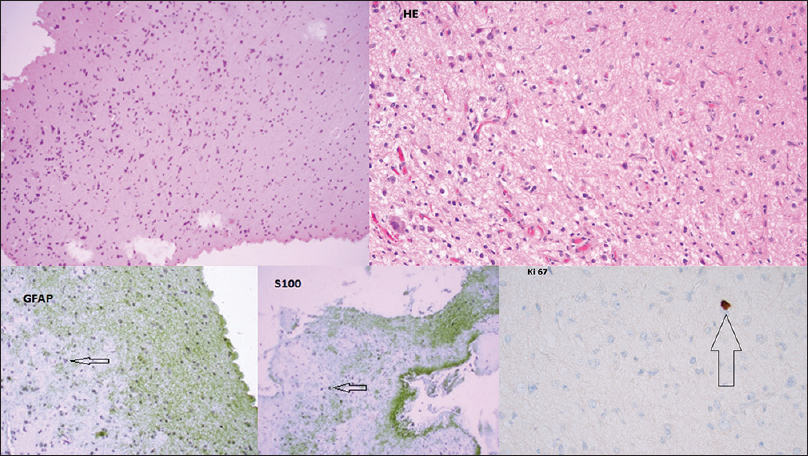
Top left: HE (Magnification ×200); DNT simple form with oligodendroglial-like cells. Top right: GFAP (Magnification ×400); immunostaining GFAP is negative in tumour cells. Bottom left: S100 (Magnification ×400); immunostaining S100 is positive in tumour cells. Bottom right: Ki-67 (Magnification ×600); immunostaining Ki-67 proliferative index is low <1%
Histopathological analysis confirmed the tumor to be a DNT of simple form without malignant transformation. After the second surgery, the patient developed a right-sided spastic palsy, and in later years, signs of cognitive dysfunction, especially with respect to learning skills. The seizures gradually diminished in frequency and intensity, reaching an ENGEL Epilepsy Surgery Outcome Scale (ES) of 2 in 2003, although the patient remained on AEDs. Follow-up MRI in January 2003 [Figure
Case 2
A 27-year-old male had developed intermittent numbness and right-sided neuralgia with upper limb predominance at the age of 11 (1999). A diagnostic MRI demonstrated a 3 cm mass with asymmetrical, nodular, and curvilinear contrast enhancement located in the left postcentral gyrus with an appearance deemed at the time consistent with a low-grade glial tumor. The EEG revealed an epileptic focus in the left frontal-temporal regions. AEDs proved ineffective for seizure control. A functional MRI located the hand motor cortex anterior to the tumor. The patient underwent gross total resection (GTR) in 1999. The initial microscopic evaluation could not differentiate between pilocytic astrocytoma and DNT. Further re-examination based on immunohistochemistry and molecular analyses (MA) proved the lesion to be a complicated type of DNT, displaying mosaic-like patterns composed of complex structures together with nonspecific elements (low-grade glioma-like structures, see
Figure 4
Top left: HE (Magnification ×200); DNT complex form tumour pattern resembling low grade glioma with multicystic (pilocytic like) pattern. Top right: GFAP (Magnification ×400); immunostaining GFAP is negative in tumour cells. Bottom left: S100 (Magnification ×400); immunostaining S100 is positive in tumour cells. Bottom right: Ki-67 (Magnification ×600); immunostaining Ki-67 proliferative index is low <1%
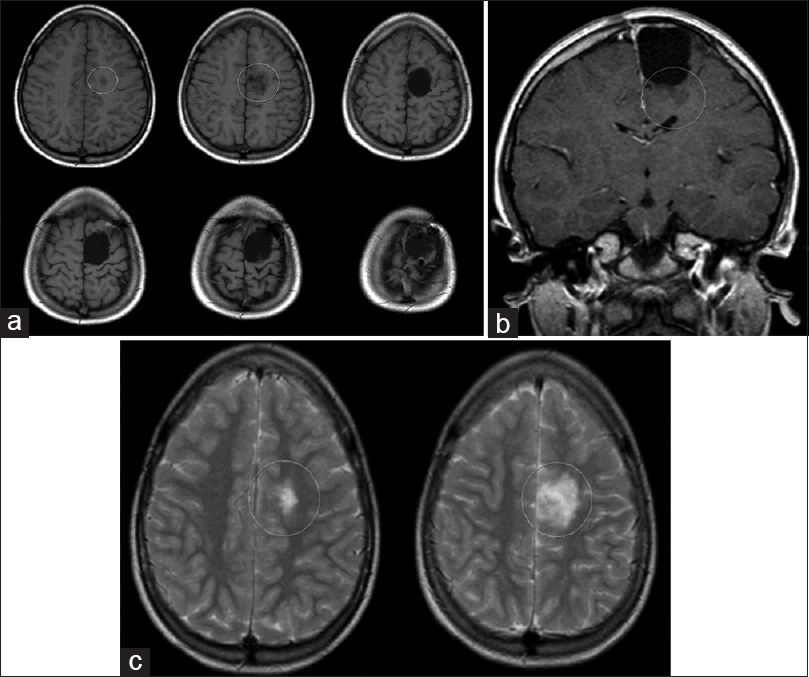
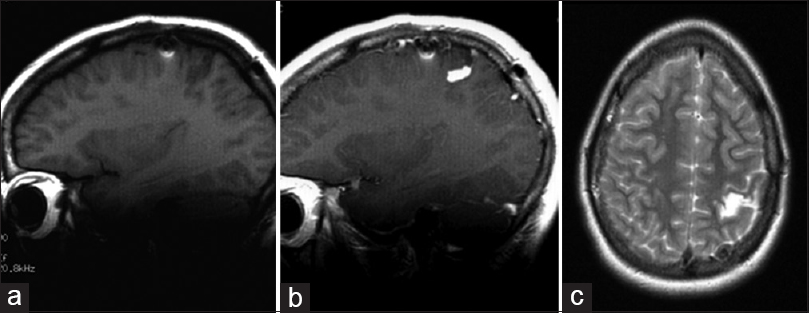
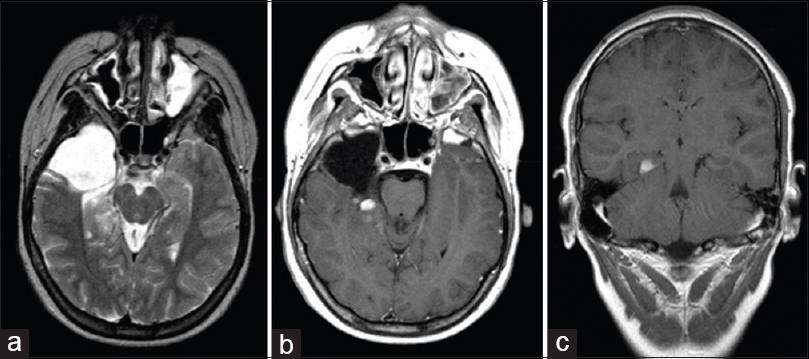
The seizures ceased almost immediately after surgery (ES 1). Postsurgical MRI demonstrated a nonenhancing residual tumor. Two years after surgery, the sensory seizures recurred. A follow-up MRI showed a new contrast-enhancing lesion within the surgical margins. Open surgery was assessed to be highly hazardous due to the tumor's close relation to adjacent motor fibers. GKRS was deemed to be the best treatment alternative. The patient was treated in 2002 with a peripheral dose of 12 Gy at the 50% isodose line [Figure
Figure 6
(a and b) T1 + contrast (left), T2 (centre) and FLAIR (right) MR images. Top row (MRI 2008) demonstrates area of T2/FLAIR high signal with no contrast enhancement in the left post central gyrus. Subsequently unchanged over 5 years of follow-up, here compared to MR images from 2013 (bottom row)
Case 3
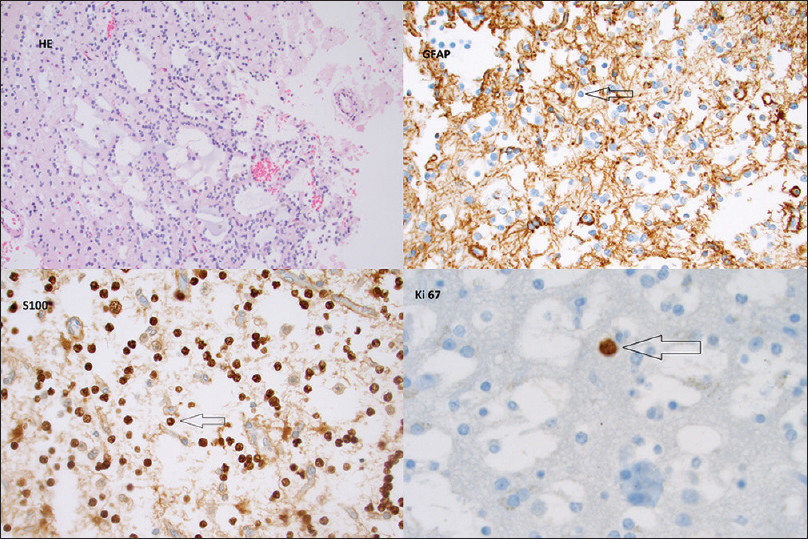
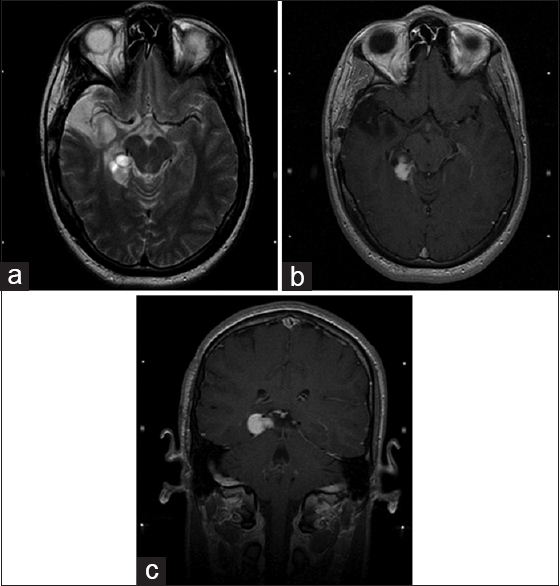
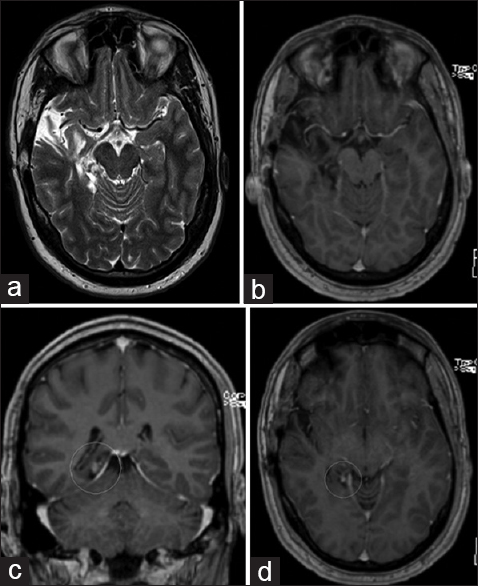
A 28-year-old male had developed complex partial seizures at the age of 6. MRI (without IV contrast) revealed a T1 hypointense/T2 hyperintense, well-delineated, possibly multilobular mass located in the inferomedial aspect of the right temporal lobe including hippocampus [Figure
In this case, we could not find evidence of prior diagnostic EEG. The patient underwent STR in 1994 and, despite the subtotal nature of the resection, seizures ceased shortly after (ES 1) and AEDs were discontinued. The histopathological analysis revealed a DNT of complex form with specific glioneuronal elements and associated FCD [
Figure 8
Top left: HE (Magnification ×100); DNT complex form with specific glioneuronal element. Top right: HE (Magnification ×400); DNT complex form with specific glioneuronal element. Bottom left: GFAP (Magnification ×200); immunostaining GFAP is negative in tumour cells. Bottom centre: S100 (Magnification ×200); immunostaining S100 is positive in tumour cells. Bottom right: Ki-67 (Magnification ×600); immunostaining Ki-67 proliferativ index is low <1%
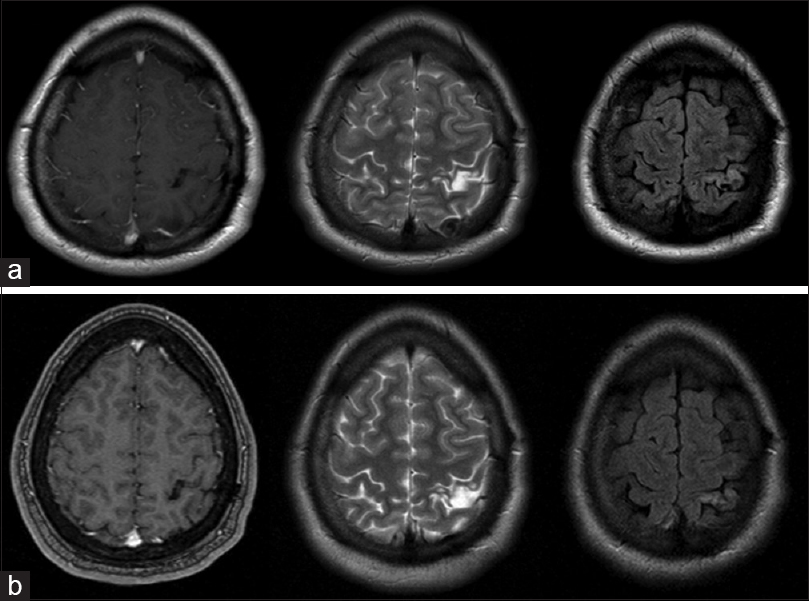
The patient remained stable until 2001, when a follow-up MRI demonstrated a suspect nodular contrast enhancement in the region of the residual tumor located in the right hippocampus and posterior aspect of the medial temporal lobe [Figure
DISCUSSION
As illustrated by our case series, DNTs might have a more complex and complicated evolution than once originally thought. The diagnostic workup of DNTs is multidisciplinary and often quite demanding. The simpler forms (described below) may be easier to diagnose due to their more distinctive histopathologic traits and underlying imaging. Even in these cases, misdiagnoses may still occur,[
Histopathology
DNTs are composed of glioneuronal elements (columns of axons lined by uniform oligodendroglioma-like cells) with intervening floating cortical neurons in mucin pools. Rare mitotic figures are also commonly found. The heterogeneous appearance of these tumors is due to the presence of astrocytic, oligodendrocytic, and neuronal components. Histologically, they are classified as simple form (specific glioneuronal elements consisting of small/round monotonous cells, so-called oligodendroglia-like cells), complex form (presence of glial nodules with internodular-specific glioneuronal elements and FCD/adjacent cortical dysplasia), and nonspecific form (unspecified glioneuronal elements, low-grade glioma-like features).[
BRAF analysis is also included in MA profiling. A study by Chappé et al.[
The expression of CD34 in DNTs has been variously reported.[
Imaging
The diagnostic, pre- and postsurgical follow-up neuroimaging is mainly based on MRI. DNTs are classified as WHO Grade I under neuronal and mixed neuronal glial tumors under tumors of the central nervous system.[
Neurophysiology
Focal (dyscognitive) seizures with impairment of consciousness (formerly complex partial seizures) are the main epileptic manifestation of DNT, followed by generalized tonic-clonic, focal motor (formerly simple partial seizures), and focal seizures evolving to bilateral convulsive seizures (formerly secondarily generalized tonic-clonic seizures).[
Microsurgery
A quantitative and comprehensive systematic literature review of seizure outcome after surgical resection of epileptogenic glioneuronal tumors (including a total of 910 patients from 39 studies) by Englot et al.[
A review on long-term epilepsy associated tumors (including glioneuronal tumors) by Giulioni et al. (publ 2014) drew similar conclusions: early tailored epilepsy surgery aims to obtain seizure freedom and avoid the potential side effects of prolonged AED as well as the risk for local recurrence and possible malignant transformation.[
A recent published review by Bonney et al. (publ 2015) covering 29 articles on seizure outcome after varyingly performed DNT surgery showed a median postsurgery seizure-free rate of 86% (though one study reported < 60% seizure freedom rate). Four studies concluded seizure freedom to be associated with more extensive surgeries. The number of seizure-free patients who discontinued AEDs varied widely.[
However, as pointed out previously, cases of local recurrence and histological evolution/malignant transformation of DNT have been reported;[
A review of 51 documented DNT cases by Daghistani et al. (2013) showed that 6 of 18 patients with STR (33.3%) presented further tumor enlargement on their follow-up imaging, while 3 of 30 patients with GTR (10%) developed tumor recurrence at the surgical bed. The group even described two cases developing secondary lesions distant to the primary site.[
Radiosurgery
The role of radiosurgery in DNT cases remains unclear, perhaps because of unreported cases. Kwon et al. (2006) reported a case of a nonresectable epileptogenic DNT lesion in the right parahippocampal gyrus treated with GKRS.[
Although still a vivid subject of discussion, some centers currently use epilepsy radiosurgery to treat recurrent/residual mesial temporal lobe epilepsy with various results.[
Finally, the management of malignant transformation of DNT is rather complex and might, depending on the case, require a more conventional management, including surgery, chemotherapy, and radiation.[
CONCLUSION
Traditionally, DNTs have been considered to be epileptogenic glioneuronal tumors with rather stable evolution after open surgery; however, recent publications are showing otherwise. In this material, GKRS resulted in long-lasting seizure-freedom (ES 1) in all cases, with two of them (Cases 1 and 3) remaining on prophylactic AED, with no observed GKRS-associated neurological deficits or cognitive dysfunction. Follow-up MRI on a regular basis has demonstrated no progression of the lesion and no ARE. In our experience, delivering 10–12 Gy to 50% isodose surface seems to be an efficient and safe strategy.
DNTs might prove difficult to diagnose; a comprehensive neurophysiological assessment, thorough neuroimaging, and complete histopathologic analysis should be included in the identification of DNTs. Surgery remains the treatment of choice and is usually performed to achieve seizure control; early surgical intervention appears to be associated with better ENGEL class outcome. Some cases of recurrence (with and without malignant transformation) have been reported in recent years. Despite access to advanced diagnostic technology and modern surgical tools, GTR is not always possible. The above may suggest the use of GKRS as a complementary, adjunctive treatment to STR (regardless of postsurgical ENGEL score outcome) and as salvage modality in cases of DNT recurrence (with or without refractory seizures) where reoperation (STR/GTR) is deemed unachievable or contraindicated. Prospective studies are warranted to establish the role of GKRS in the treatment of DNTs.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. A. Nicolato ML, Foroni R, Alessandrini F, De Simone A, Ghimenton C, De Carlo A, Mirtuono P, Gerosa M, Mathieu D. Gamma knife radiosurgery in the management of unusual grade I/II primitive neuroepithelial tumours of the brain. Gamma Knife Radiosurgery: InTech. 2011. p. 73-100
2. Aggarwal A, Salunke P, Sodhi HB, Vasishta RK, Gowda KK. Dysembryoplastic neuroepithelial tumor transforming into malignancy: A case report. Neurol India. 2014. 62: 323-5
3. Blumcke I, Aronica E, Urbach H, Alexopoulos A, Gonzalez-Martinez JA. A neuropathology-based approach to epilepsy surgery in brain tumors and proposal for a new terminology use for long-term epilepsy-associated brain tumors. Acta Neuropathol. 2014. 128: 39-54
4. Bodi I, Selway R, Bannister P, Doey L, Mullatti N, Elwes R. Diffuse form of dysembryoplastic neuroepithelial tumour: The histological and immunohistochemical features of a distinct entity showing transition to dysembryoplastic neuroepithelial tumour and ganglioglioma. Neuropathol Appl Neurobiol. 2012. 38: 411-25
5. Bonney PA, Boettcher LB, Conner AK, Glenn CA, Briggs RG, Santucci JA. Review of seizure outcomes after surgical resection of dysembryoplastic neuroepithelial tumors. J Neurooncol. 2016. 126: 1-10
6. Bulakbasi N, Kocaoglu M, Sanal TH, Tayfun C. Dysembryoplastic neuroepithelial tumors: Proton MR spectroscopy, diffusion and perfusion characteristics. Neuroradiology. 2007. 49: 805-12
7. Campos AR, Clusmann H, von Lehe M, Niehusmann P, Becker AJ, Schramm J. Simple and complex dysembryoplastic neuroepithelial tumors (DNT) variants: Clinical profile, MRI, and histopathology. Neuroradiology. 2009. 51: 433-43
8. Chang EF, Christie C, Sullivan JE, Garcia PA, Tihan T, Gupta N. Seizure control outcomes after resection of dysembryoplastic neuroepithelial tumor in 50 patients. J Neurosurg Pediatr. 2010. 5: 123-30
9. Chao L, Tao XB, Jun YK, Xia HH, Wan WK, Tao QS. Recurrence and histological evolution of dysembryoplastic neuroepithelial tumor: A case report and review of the literature. Oncol Lett. 2013. 6: 907-14
10. Chappe C, Padovani L, Scavarda D, Forest F, Nanni-Metellus I, Loundou A. Dysembryoplastic neuroepithelial tumors share with pleomorphic xanthoastrocytomas and gangliogliomas BRAF(V600E) mutation and expression. Brain Pathol. 2013. 23: 574-83
11. Chuang NA, Yoon JM, Newbury RO, Crawford JR. Glioblastoma multiforme arising from dysembryoplastic neuroepithelial tumor in a child in the absence of therapy. J Pediatr Hematol Oncol. 2014. 36: e536-9
12. Consales A, Striano P, Nozza P, Morana G, Ravegnani M, Piatelli G. Glioneuronal tumors and epilepsy in children: Seizure outcome related to lesionectomy. Minerva Pediatr. 2013. 65: 609-16
13. Daghistani R, Miller E, Kulkarni AV, Widjaja E. Atypical characteristics and behavior of dysembryoplastic neuroepithelial tumors. Neuroradiology. 2013. 55: 217-24
14. Daumas-Duport C, Scheithauer BW, Chodkiewicz JP, Laws ER, Vedrenne C. Dysembryoplastic neuroepithelial tumor: A surgically curable tumor of young patients with intractable partial seizures. Report of thirty-nine cases. Neurosurgery. 1988. 23: 545-56
15. Durnford AJ, Rodgers W, Kirkham FJ, Mullee MA, Whitney A, Prevett M. Very good inter-rater reliability of Engel and ILAE epilepsy surgery outcome classifications in a series of 76 patients. Seizure. 2011. 20: 809-12
16. Eksi MS, Yilmaz B, Akakin A, Toktas ZO, Kaur AC, Demir MK. Gamma knife treatment of low-grade gliomas in children. Childs Nerv Syst. 2015. 31: 2015-23
17. Eman Abdelzaher MDLast updated on 2013 Dec 14; Last cited on 2016 Apr 01. Available from: http://www.pathologyoutlines.com/topic/cnstumorDNET.html .
18. Englot DJ, Berger MS, Barbaro NM, Chang EF. Factors associated with seizure freedom in the surgical resection of glioneuronal tumors. Epilepsia. 2012. 53: 51-7
19. Englot DJ, Berger MS, Barbaro NM, Chang EF. Predictors of seizure freedom after resection of supratentorial low-grade gliomas. A review. J Neurosurg. 2011. 115: 240-4
20. Garcia-Fernandez M, Fournier-Del Castillo C, Ugalde-Canitrot A, Perez-Jimenez A, Alvarez-Linera J, De Prada-Vicente I. Epilepsy surgery in children with developmental tumours. Seizure. 2011. 20: 616-27
21. Giulioni M, Marucci G, Martinoni M, Marliani AF, Toni F, Bartiromo F. Epilepsy associated tumors: Review article. World J Clin Cases. 2014. 2: 623-41
22. Kwon KH, Lee JI, Hong SC, Seo DW, Hong SB. Gamma knife radiosurgery for epilepsy related to dysembryoplastic neuroepithelial tumor. Stereotact Funct Neurosurg. 2006. 84: 243-7
23. Lee EM, Kang JK, Kim SJ, Hong SH, Ko TS, Lee SA. Gamma knife radiosurgery for recurrent or residual seizures after anterior temporal lobectomy in mesial temporal lobe epilepsy patients with hippocampal sclerosis: Long-term follow-up results of more than 4 years. J Neurosurg. 2015. 123: 1375-82
24. Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007. 114: 97-109
25. Maher CO, White JB, Scheithauer BW, Raffel C. Recurrence of dysembryoplastic neuroepithelial tumor following resection. Pediatr Neurosurg. 2008. 44: 333-6
26. Mano Y, Kumabe T, Shibahara I, Saito R, Sonoda Y, Watanabe M. Dynamic changes in magnetic resonance imaging appearance of dysembryoplastic neuroepithelial tumor with or without malignant transformation. J Neurosurg Pediatr. 2013. 11: 518-25
27. Maris D, Nica D, Mohan D, Moisa H, Ciurea AV. Multidisciplinary management of adult low grade gliomas. Chirurgia (Bucur). 2014. 109: 590-9
28. Marti Fuster B, Esteban O, Planes X, Aguiar P, Crespo C, Falcon C. FocusDET, a new toolbox for SISCOM analysis. Evaluation of the registration accuracy using Monte Carlo simulation. Neuroinformatics. 2013. 11: 77-89
29. Martinoni M, Marucci G, Rubboli G, Volpi L, Riguzzi P, Marliani F. Focal cortical dysplasias in temporal lobe epilepsy surgery: Challenge in defining unusual variants according to the last ILAE classification. Epilepsy Behav. 2015. 45: 212-6
30. Matsuda H, Matsuda K, Nakamura F, Kameyama S, Masuda H, Otsuki T. Contribution of subtraction ictal SPECT coregistered to MRI to epilepsy surgery: A multicenter study. Ann Nucl Med. 2009. 23: 283-91
31. Moazzam AA, Wagle N, Shiroishi MS. Malignant transformation of DNETs: A case report and literature review. Neuroreport. 2014. 25: 894-9
32. Murakami N, Morioka T, Hashiguchi K, Suzuki SO, Shigeto H, Sakata A. Clinical and histological characteristics of ictal onset zone in cases of intractable epilepsy associated with dysembryoplastic neuroepithelial tumor. Brain Nerve. 2015. 67: 525-32
33. Paudel K, Borofsky S, Jones RV, Levy LM. Dysembryoplastic neuroepithelial tumor with atypical presentation: MRI and diffusion tensor characteristics. J Radiol Case Rep. 2013. 7: 7-14
34. Penagaricano J, Serletis D. Radiosurgery in the management of intractable mesial temporal lobe epilepsy. J Ark Med Soc. 2015. 112: 66-7
35. Ray WZ, Blackburn SL, Casavilca-Zambrano S, Barrionuevo C, Orrego JE, Heinicke H. Clinicopathologic features of recurrent dysembryoplastic neuroepithelial tumor and rare malignant transformation: A report of 5 cases and review of the literature. J Neurooncol. 2009. 94: 283-92
36. Rheims S, Rubi S, Bouvard S, Bernard E, Streichenberger N, Guenot M. Accuracy of distinguishing between dysembryoplastic neuroepithelial tumors and other epileptogenic brain neoplasms with [(1)(1) C] methionine PET. Neuro Oncol. 2014. 16: 1417-26
37. Santos MV, de Oliveira RS, Machado HR. Approach to cortical dysplasia associated with glial and glioneuronal tumors (FCD type IIIb). Childs Nerv Syst. 2014. 30: 1869-74
38. Sayuthi S, Tharakan J, George J, Pieter MS, Salmah WM, Madhavan M. Epilepsy surgery on dysembryoplastic neuroepithelial tumours. Med J Malaysia. 2006. 61: 374-6
39. Song JY, Kim JH, Cho YH, Kim CJ, Lee EJ. Treatment and outcomes for gangliogliomas: A single-center review of 16 patients. Brain Tumor Res Treat. 2014. 2: 49-55
40. Suh YL. Dysembryoplastic neuroepithelial tumors. J Pathol Transl Med. 2015. 49: 438-49
41. Takeuchi Y, Arakawa Y, Mikami Y, Matsumoto R, Miyamoto S. Dysembryoplastic neuroepithelial tumor with rapid recurrence of pilocytic astrocytoma component. Brain Tumor Pathol. 2014. 31: 144-8
42. Tanaka S, Shin M, Mukasa A, Hanakita S, Saito K, Koga T. Stereotactic radiosurgery for intracranial gliomas. Neurosurg Clin N Am. 2013. 24: 605-12
43. Tarapore PE, Picht T, Bulubas L, Shin Y, Kulchytska N, Meyer B. Safety and tolerability of navigated TMS for preoperative mapping in neurosurgical patients. Clin Neurophysiol. 2016. 127: 1895-900
44. Thom M, Toma A, An S, Martinian L, Hadjivassiliou G, Ratilal B. One hundred and one dysembryoplastic neuroepithelial tumors: An adult epilepsy series with immunohistochemical, molecular genetic, and clinical correlations and a review of the literature. J Neuropathol Exp Neurol. 2011. 70: 859-78
45. Vale FL, Bozorg AM, Schoenberg MR, Wong K, Witt TC. Long-term radiosurgery effects in the treatment of temporal lobe epilepsy. J Neurosurg. 2012. 117: 962-9
46. Vojtech Z, Malikova H, Syrucek M, Kramska L, Sroubek J, Vladyka V. Morphological changes after radiosurgery for mesial temporal lobe epilepsy. Acta Neurochir (Wien). 2015. 157: 1783-91
47. von Lehe M, Lutz M, Kral T, Schramm J, Elger CE, Clusmann H. Correlation of health-related quality of life after surgery for mesial temporal lobe epilepsy with two seizure outcome scales. Epilepsy Behav. 2006. 9: 73-82
48. Willemse RB, Hillebrand A, Ronner HE, Vandertop WP, Stam CJ. Magnetoencephalographic study of hand and foot sensorimotor organization in 325 consecutive patients evaluated for tumor or epilepsy surgery. Neuroimage Clin. 2016. 10: 46-53
49. Xue H, Sveinsson O, Li YJ. Resection of a dysembryoplastic neuroepithelial tumor in the precentral gyrus. World J Pediatr. 2015. 11: 281-3