- Department of Neurosurgery, Kyushu Rosai Hospital, Kitakyushu, Japan
- Department of Cerebrovascular Disease, Kyushu Rosai Hospital, Kitakyushu, Japan
- Department of Neurosurgery, Graduate School of Medical Sciences, Kyushu University, Fukuoka, Japan
- Department of Neurosurgery, Fukuoka Children's Hospital, Fukuoka, Japan
Correspondence Address:
Takafumi Shimogawa
Department of Neurosurgery, Kyushu Rosai Hospital, Kitakyushu, Japan
Department of Neurosurgery, Graduate School of Medical Sciences, Kyushu University, Fukuoka, Japan
DOI:10.4103/sni.sni_73_17
Copyright: © 2017 Surgical Neurology International This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Takafumi Shimogawa, Takato Morioka, Tomoaki Akiyama, Sei Haga, Shuji Arakawa, Tetsuro Sayama. Sequential changes of arterial spin-labeling perfusion MR images with dual postlabeling delay following reconstructive surgery for giant internal carotid artery aneurysm. 07-Sep-2017;8:222
How to cite this URL: Takafumi Shimogawa, Takato Morioka, Tomoaki Akiyama, Sei Haga, Shuji Arakawa, Tetsuro Sayama. Sequential changes of arterial spin-labeling perfusion MR images with dual postlabeling delay following reconstructive surgery for giant internal carotid artery aneurysm. 07-Sep-2017;8:222. Available from: http://surgicalneurologyint.com/surgicalint-articles/sequential-changes-of-arterial-spin%e2%80%91labeling-perfusion-mr-images-with-dual-postlabeling-delay-following-reconstructive-surgery-for-giant-internal-carotid-artery-aneurysm/
Abstract
Background:Arterial spin-labeling magnetic resonance perfusion imaging (ASL-MRI) allows noninvasive measurement of cerebral blood flow (CBF) but depends on arterial transit time (ATT). To overcome this problem, we developed a simple ASL technique with dual postlabeling delay (PLD) settings. In addition to the routinely used PLD of 1.5 seconds, we selected another PLD of 2.5 seconds to assess slowly streaming blood flow and detect arterial transit artifacts (ATAs) resulting from stagnant intravascular magnetically labeled spins.
Case Description:We validated the dual PLD method with digital subtraction angiography (DSA) findings in a patient with an unruptured right giant internal carotid artery (ICA) aneurysm who underwent proximal ligation of the right cervical ICA followed by right superficial temporal artery–middle cerebral artery anastomosis. The giant aneurysm was detected as a strongly hyperintense signal area of ATA using both values of PLD. Decreased signal in the right hemisphere at PLD 1.5 seconds was somewhat improved at PLD 2.5 seconds. DSA revealed that this laterality resulted from the different ATT values between hemispheres due to stagnation of the labeled spin within the aneurysm. Postoperatively, with gradual but complete thrombosis and regression of the aneurysm, the size of the ASL hyperintense signal area decreased markedly. At postoperative 2 years, the aneurysm was not demonstrated as an ATA; furthermore, the decreased signals in the right hemisphere at PLD 1.5 seconds had mostly improved.
Conclusion:Serial ASL-MRI with dual PLDs could show dynamic changes of giant aneurysms and the associated hemodynamic state following the surgery.
Keywords: Arterial spin-labeling perfusion MR imaging, arterial transit artifact, arterial transit time, giant aneurysm, postlabeling delay
INTRODUCTION
Arterial spin-labeling magnetic resonance perfusion imaging (ASL-MRI) visualizes cerebral perfusion using magnetically labeled protons in arterial blood as an endogenous tracer.[
In patients with steno-occlusive cerebrovascular disease, including leptomeningeal anastomosis, labeled blood that travels via collateral pathways of the circle of Willis or secondary collateral pathways exhibits prolonged ATT.[
Another drawback of ASL-MRI is that, in patients with well-developed collaterals, a single conventional PLD shows apparent hyperperfusion in territories where intravascular magnetically labeled protons is stagnant, a condition termed “arterial transit artifact (ATA).”[
In this study, we examined the usefulness of ASL-MRI with dual PLDs to elucidate sequential changes in response to thrombosis and regression of the giant aneurysm and alteration of the associated hemodynamic state following reconstructive surgery. These ASL findings were validated with DSA findings according to the methods of our previous reports.[
CASE REPORT
A woman aged 66 years presented with decreased right visual acuity over several months. On admission, her visual acuity was 0.04 on the right and 0.1 on the left. Right abducens palsy was noted. Conventional MRI and MR angiography (MRA) revealed a giant right ICA aneurysm (diameter 30 mm) and a left ICA aneurysm (5 mm) [Figure
Figure 1
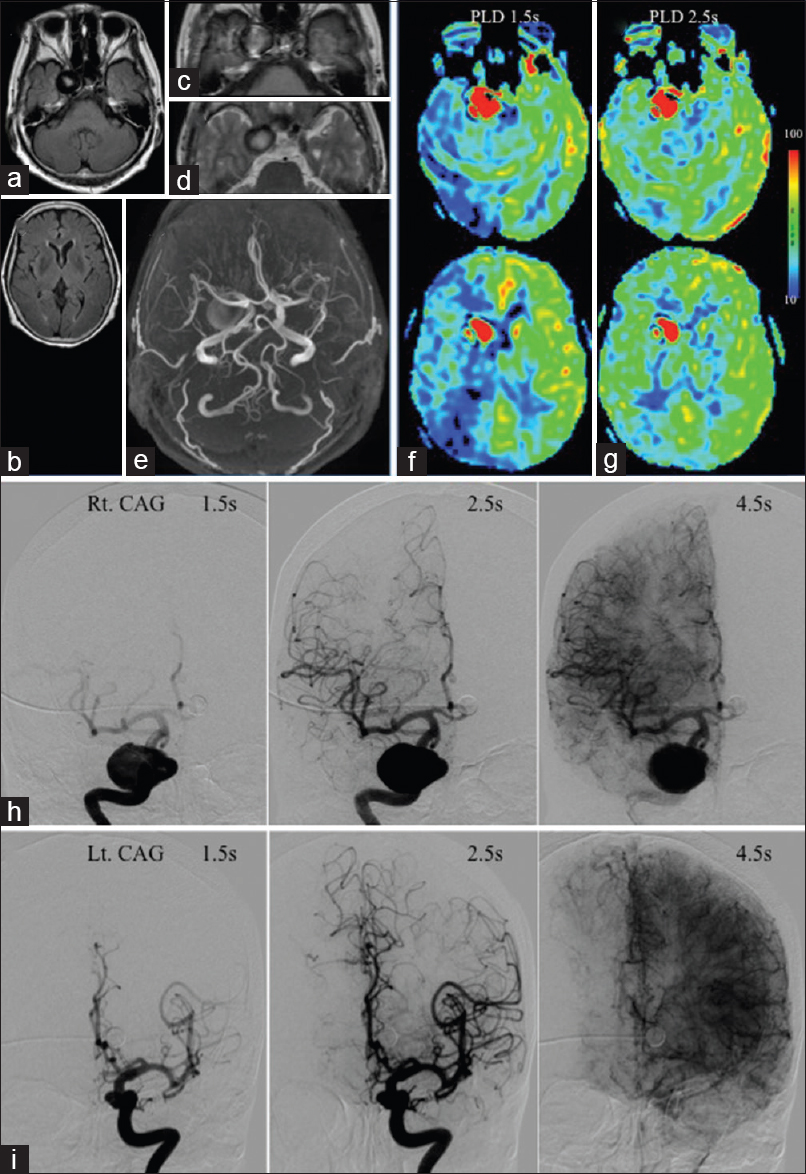
MRIs (a-d) and MRA (e) show right giant and left ICA aneurysms. Both on ASL with PLDs of 1.5 (f) and 2.5 s (g), the right aneurysm is depicted as hyperintense signal in the same configuration. ASL with PLD 1.5 s reveals decreased signal in the right MCA territory, and with 2.5 s improved. (h) Right CAG shows the contrast in the right aneurysm is stagnant at 4.5 s after injection. At 1.5 s, the trunks of the right MCA and ACA are barely visualized because of stagnation, and are opacified at 2.5 s. (i) Left CAG shows that the trunks of the left MCA and ACA are well opacified at 1.5 s
In ASL-MRI with PLDs of 1.5 and 2.5 seconds, although the left aneurysm was not demonstrated, the right ICA aneurysm was depicted as a hyperintense signal area in nearly the same configuration [Figure
Carotid angiography (CAG) at 1.5 and 2.5 seconds after contrast medium injection into the right and left ICA, respectively, clearly demonstrated the nonthrombosed giant aneurysm at the cavernous portion of the right ICA [
The balloon Matas and Allcock test revealed robust collateral flows through the anterior communicating artery (ACoA) and the right posterior communicating artery (PCoA), respectively. During 20 minutes of balloon test occlusion (BTO), her neurological findings and somatosensory evoked potentials (SEPs) following left median nerve stimulation did not show any decline. For the treatment of the “symptomatic” giant right IC aneurysm, proximal ligation of the right cervical ICA was indicated. The patient underwent right superficial temporal artery (STA)-MCA bypass just before the proximal ICA ligation on the same day with monitoring of SEPs and transcranial motor evoked potentials.
During postoperative (PO) 2 months, conventional MRI revealed a gradual signal change of the right aneurysm, although the aneurysm's size had not changed. T1WI depicted the aneurysm as homogenous and iso–low-intensity at PO 1 day [
Figure 2
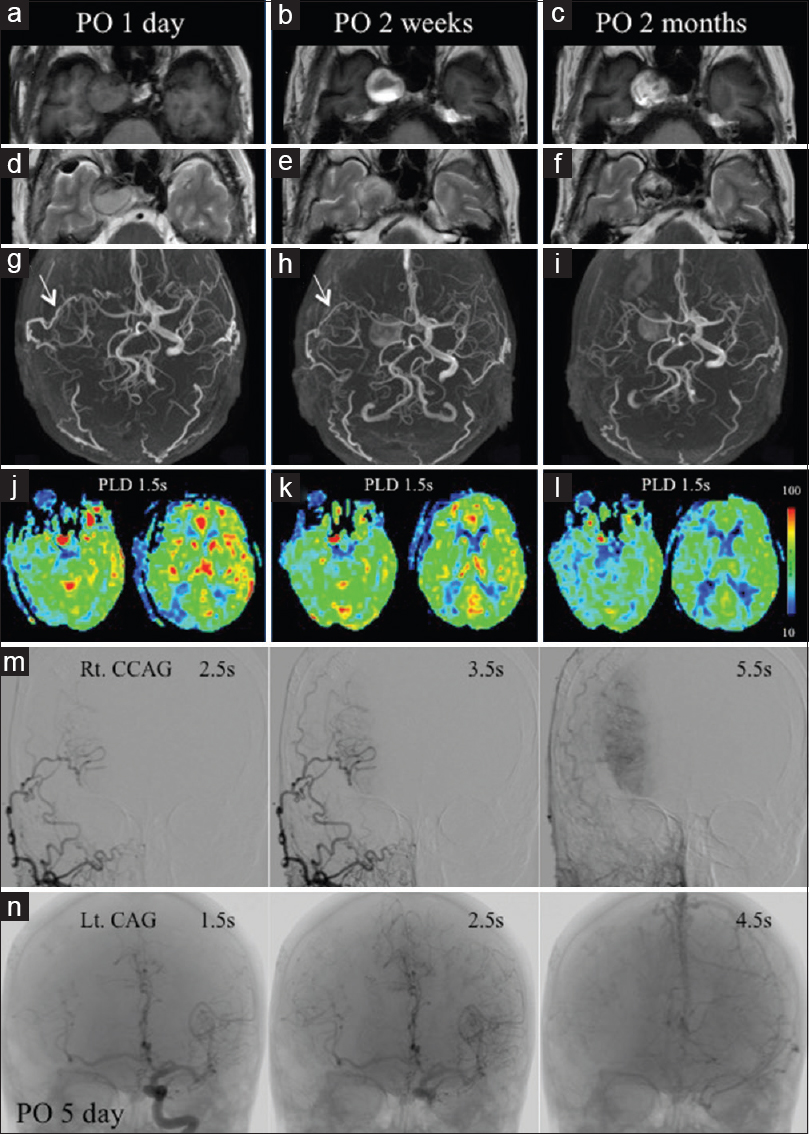
MRIs show gradual signal change in the right aneurysm, although the size is unchanged (a-f). The aneurysm is not demonstrable on MRA at postoperative (PO) 1 day (g), but reappears at PO 2 weeks and 2 months (h, i). The bypass is patent at PO 1 day and 2 weeks (g, h), but not at 2 months (i). Perioperative ASL (j-l) shows a gradual decrease in aneurysm size and improving ASL signals in the right MCA territory. Right common CAG at PO 5 days (m) shows part of the right MCA visualized at 2.5 and 3.5 s. Left CAG (n) reveals the trunk and periphery of the right MCA are visualized at 1.5 and 2.5 s
Perioperative ASL-MRI with a PLD of 1.5 seconds demonstrated a gradual decrease in the size of the aneurysm. At PO 1 day, the size of the ASL hyperintense signal area had markedly decreased in size compared with that shown by preoperative ASL-MRI, and the hyperintense signal area was not demonstrated at the level of the basal ganglia [
DSA was performed at PO 5 days. Right common CAG (CCAG) demonstrated complete patency of the bypass, and part of the right MCA territory was visualized through this bypass at 2.5 and 3.5 seconds (equivalent to 1.5 and 2.5 seconds in CAG, respectively) [
At PO 1 year, the patient had completely recovered from her right visual disturbance and abducens palsy and underwent coil embolization of the left ICA aneurysm. At that time, right CCAG failed to reveal the patency of the bypass [
Figure 3
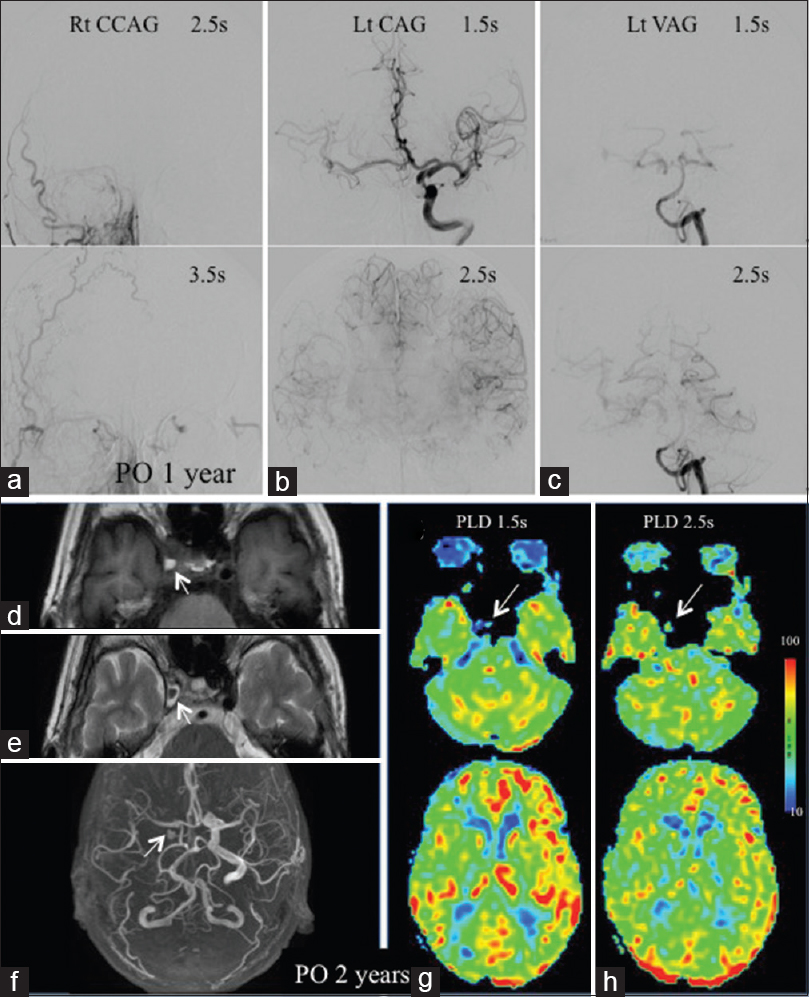
(a) Right common CAG at PO 1 year reveals no patency of the bypass. (b) The right MCA trunk and periphery are visualized at 1.5 and 2.5 s on left CAG. (c) Left vertebral angiography depicts right MCA periphery visualization at 2.5 s. MRIs (d-f) at PO 2 years reveal a marked size decrease of the right aneurysm. ASL shows the aneurysm as a tiny spot of low signal at 1.5 s (g) and iso at 2.5s (h). ASL with PLD 1.5 s shows the preoperative decreased ASL signals in the right MCA territory improving with slight laterality (g). ASL with PLD 2.5 s shows this laterality improving somewhat (h)
At PO 2 years, conventional MRI and MRA demonstrated a marked size decrease of the right ICA aneurysm. The aneurysm was detected as a small hyperintensity surrounded by a low-intensity region in T1WI [
ASL-MRI with dual PLDs demonstrated the aneurysm as a tiny spot of low-intensity signal at PLD 1.5 seconds [
DISCUSSION
ASL signal changes of the giant aneurysm
The prominent finding of preoperative ASL-MRI was that the right giant ICA aneurysm was detected as a hyperintense signal area, while the aneurysm itself was generally not demonstrated on ASL-MRI (as seen for the left aneurysm). The hyperintense signal area of the right giant aneurysm remained in nearly the same configuration in ASL-MRI with PLDs of 1.5 and 2.5 seconds, indicating that the aneurysm demonstrated an ATA.[
The giant aneurysm presented large ATAs resulting from the swirl of the strongly labeled spins in the stagnating blood inside the aneurysm, which extended to the axial level of the basal ganglia. We have also reported a patient with ICA stenosis in whom round-shaped ATAs of delayed and stagnant anterograde flow extended to the level of the basal ganglia.[
At PO 2 months, conventional MRI revealed gradual signal change of the aneurysm, although its size had not changed. According to previous reports,[
In contrast to conventional MRI, ASL-MRI with PLD 1.5 seconds at PO 2 months showed a markedly and gradually decreased size of the aneurysmal hyperintense signal area. Furthermore, no concentric artifact was noted. Without the availability of ASL-MRI data with PLD 2.5 seconds, whether the ASL-MRI's hyperintense signal is attributed to the ATA cannot be confirmed; however, it is speculated that labeled flow spins dramatically decreased in association with the flow reduction and staged aneurysmal thrombosis.
At PO 2 years, ASL-MRI with dual PLDs demonstrated the aneurysm as a tiny low-intensity spot at 1.5 seconds and an iso-signal region at 2.5 seconds. The intensity and configuration of the spot were not identical between PLDs of 1.5 and 2.5 seconds, indicating that this area did not present as ATA but as blood flow near the aneurysmal wall and vasa vasorum.[
ASL signals changes to associated hemodynamic states
Preoperative ASL-MRI with PLD 1.5 seconds demonstrated decreased signals in the right hemisphere, especially the right MCA territory. ASL-MRI with PLD 2.5 seconds showed improvement in this decreased-signal area. DSA revealed that this laterality of ASL signals resulted not from the different CBF values but from the different ATT values between hemispheres; this effect was caused by stagnation of contrast within the aneurysm and delayed anterograde flow, as demonstrated in our previous reports.[
At PO 1 day, ASL signals at PLD 1.5 seconds increased across both hemispheres compared with those shown in preoperative ASL-MRI; furthermore, at that time, the preoperative decreased ASL signals in the right hemisphere were markedly improved. Because no perioperative ASL-MRI data were available with dual PLDs, these findings are speculated to have resulted from the shortened ATT[
At PO 2 years, the preoperative decreased signals in the right hemisphere were markedly improved at PLD 1.5 seconds, as stagnation of labeled spins within the aneurysm had disappeared. ASL-MRI with dual PLDs demonstrated a slightly decreased right ASL signal at 1.5 seconds, which was somewhat improved at 2.5 seconds. This was explained by the DSA findings at PO 1 year: with the obstruction of the bypass, right MCA territory were opacified with primary collateral pathway via ACoA and PCoA at 2.5 seconds. Labeled blood that travels via collateral pathways of the circle of Willis exhibit prolonged ATT as long as 1 second.[
In this case, the bypass was spontaneously occluded in the postoperative course without any sequels. It implies that the patient was tolerant for ICA occlusion without bypass, as was expected by preoperative BTO findings. Since there have been reports describing the development of the postoperative ischemic complications without bypass even among patients with negative BTO findings,[
CONCLUSION
Although our experience is limited to a single case, serial ASL-MRI with dual PLDs showed dynamic changes in response to thrombosis and regression of the giant aneurysm and alteration of the associated hemodynamic state following reconstructive surgery.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Akiyama T, Morioka T, Shimogawa T, Haga S, Sayama T, Kanazawa Y. Arterial spin-labeling magnetic resonance perfusion imaging with dual postlabeling delay in internal carotid artery steno-occlusion: Validation with digital subtraction angiography. J Stroke Cerebrovasc Dis. 2016. 25: 2099-108
2. Deibler AR, Pollock JM, Kraft RA, Tan H, Burdette JH, Maldjian JA. Arterial spin-labeling in routine clinical practice. Part 1. Technique and artifacts. AJNR Am J Neuroradiol. 2008. 29: 1228-34
3. Deibler AR, Pollock JM, Kraft RA, Tan H, Burdette JH, Maldjian JA. Arterial spin-labeling in routine clinical practice. Part 2. Hypoperfusion patterns. AJNR Am J Neuroradiol. 2008. 29: 1235-41
4. Deibler AR, Pollock JM, Kraft RA, Tan H, Burdette JH, Maldjian JA. Arterial spin-labeling in routine clinical practice. Part 3. Hyperperfusion patterns. AJNR Am J Neuroradiol. 2008. 29: 1428-35
5. Ferré JC, Bannier E, Raoult H, Mineur G, Carsin-Nicol B, Gauvrit JY. Arterial spin labeling (ASL) perfusion: Techniques and clinical use. Diagn Interv Imaging. 2013. 94: 1211-23
6. Haga S, Morioka T, Shimogawa T, Akiyama T, Murao K, Kanazawa Y. Arterial spin labeling perfusion magnetic resonance image with dual postlabeling delay: A correlative study with acetazolamide loading 123 I-Iodoanphetamine single-photon emission computed tomography. J Stroke Cerebrovasc Dis. 2016. 25: 1-6
7. Kanazawa Y, Morioka T, Arakawa S, Furuta Y, Nakanishi A, Kitazono T. Nonconvulsive partial status epilepticus mimicking recurrent infarction revealed by diffusion-weighted and arterial spin labeling perfusion magnetic resonance images. J Stroke Cerebrovasc Dis. 2015. 24: 731-8
8. Kansaku K, Hirai S, Kobayashi E, Ono J, Yamaura A. Serial magnetic resonance imaging of acute spontaneous thrombosis of a giant intracranial aneurysm. Case report. Neurol Med Chir (Tokyo). 1998. 38: 562-5
9. Lyu J, Ma N, Liebeskind DS, Wang DJ, Ma L, Xu Y. Arterial spin labeling magnetic resonance imaging estimation of antegrade and collateral flow in unilateral middle cerebral artery stenosis. Stroke. 2016. 47: 428-33
10. Martin AJ, Hetts SW, Dillon WP, Higashida RT, Halbach V, Dowd CF. MR imaging of partially thrombosed cerebral aneurysms: Characteristics and evolution. AJNR Am J Neuroradiol. 2011. 32: 346-51
11. Morioka T, Matsushima T, Fujii K, Fukui M, Hasuo K, Hisashi K. Balloon test occlusion of the internal carotid artery with monitoring of compressed spectral arrays (CSAs) of electroencephalogram. Acta Neurochir (Wien). 1989. 101: 29-34
12. Roski RA, Spetzler RF, Nulsen FE. Late complications of carotid ligation in the treatment of intracranial aneurysms. J Neurosurg. 1981. 54: 583-7
13. Shimogawa T, Morioka T, Sayama T, Haga S, Akiyama T, Murao K. Signal changes on magnetic resonance perfusion images with arterial spin labeling after carotid endarterectomy. Surg Neurol Int. 2016. 7: S1031-40
14. Teng MM, Nasir Qadri SM, Luo CB, Lirng JF, Chen SS, Chang CY. MR imaging of giant intracranial aneurysm. J Clin Neurosci. 2003. 10: 460-4
15. Wakisaka K, Morioka T, Shimogawa T, Murao K, Kanazawa Y, Hagiwara N. Epileptic ictal hyperperfusion on arterial spin labeling perfusion and diffusion-weighted magnetic resonance images in posterior reversible encephalopathy syndrome. J Stroke Cerebrovasc Dis. 2016. 25: 228-37
16. Yoo RE, Yun TJ, Rhim JH, Yoon BW, Kang KM, Choi SH. Bright vessel appearance on arterial spin labeling MRI for localizing arterial occlusion in acute ischemic stroke. Stroke. 2015. 46: 564-7
17. Zaharchuk G, Do HM, Marks MP, Rosenberg J, Moseley ME, Steinberg GK. Arterial spin-labeling MRI can identify the presence and intensity of collateral perfusion in patients with moyamoya disease. Stroke. 2011. 42: 2485-91