- Division of Neurosurgery, City of Hope, Los Angeles, USA
- Department of Neurosurgery, University of California - San Diego, San Diego, USA
- Department of Neurosurgery, Stanford University, Palo Alto, USA
- Department of Radiation Oncology, University of California - Irvine, Irvine, California, USA
Correspondence Address:
Charles L. Limoli
Department of Radiation Oncology, University of California - Irvine, Irvine, California, USA
DOI:10.4103/sni.sni_250_17
Copyright: © 2018 Surgical Neurology International This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Rahul Jandial, Reid Hoshide, J. Dawn Waters, Charles L. Limoli. Space–brain: The negative effects of space exposure on the central nervous system. 16-Jan-2018;9:9
How to cite this URL: Rahul Jandial, Reid Hoshide, J. Dawn Waters, Charles L. Limoli. Space–brain: The negative effects of space exposure on the central nervous system. 16-Jan-2018;9:9. Available from: http://surgicalneurologyint.com/surgicalint-articles/space-brain-the-negative-effects-of-space-exposure-on-the-central-nervous-system/
Abstract
Journey to Mars will be a large milestone for all humankind. Throughout history, we have learned lessons about the health dangers associated with exploratory voyages to expand our frontiers. Travelling through deep space, the final frontier, is planned for the 2030s by NASA. The lessons learned from the adverse health effects of space exposure have been encountered from previous, less-lengthy missions. Prolonged multiyear deep space travel to Mars could be encumbered by significant adverse health effects, which could critically affect the safety of the mission and its voyagers. In this review, we discuss the health effects of the central nervous system by space exposure. The negative effects from space radiation and microgravity have been detailed. Future aims and recommendations for the safety of the voyagers have been discussed. With proper planning and anticipation, the mission to Mars can be done safely and securely.
Keywords: Mars, microgravity, radiation, space travel
INTRODUCTION
Throughout the existence of humankind explorers have taken great risks to discover new land for the advancement of tribe, science, commerce, and for the fulfilment of our inherent curiosity of the unknown. From seafarers’ travels across unchartered ocean to the astronauts’ trekking across the lunar surface, we have been triumphant in exploring our world and beyond. The next feat is Mars. Despite mankind's many successes, these journeys have been at the expense of significant morbidity and mortality of the explorers. While many of these pioneers were focused on learning about the techniques of exploration, they also learned about the unanticipated health challenges that came with it. Travel to Mars could suffer the same consequences. While NASA has made significant advances in the technology and engineering to make a mission to Mars feasible, the health concerns of the astronauts during this extended journey remains a significant presentiment.
While engineers ponder and develop the technologies to take us to the farthest galaxies, the health of the astronauts is of equal if not more importance. Machines sent from earth have already successfully landed on Mars, the question remains about feasibility of human interplanetary travel. Exposure to the space environment has been shown to affect all systems of human physiology and anatomy. Countermeasures to re-establish homeostatic states within the human body have begun, but the pathophysiologic mechanisms of space exposure on the neurologic system are still obscure.
Here, we summarize the current knowledge of neurologic compromise encountered or expected from space exposure, the most foreign environmental pressure to affect the human species.
Radiation exposure
The intrigue of deep space travel comes with inherent risks, and one that has recently come to light involves exposure to the ionizing radiation fields in space. In fact, exposure to these complex radiation fields in space has been identified as the primary risk to astronaut health as they venture from the protective magnetosphere of the Earth and beyond low Earth orbit en-route to distant worlds such as Mars.[
For decades, clinical literature has informed health professionals that radiation exposure has certain unintended and adverse consequences.[
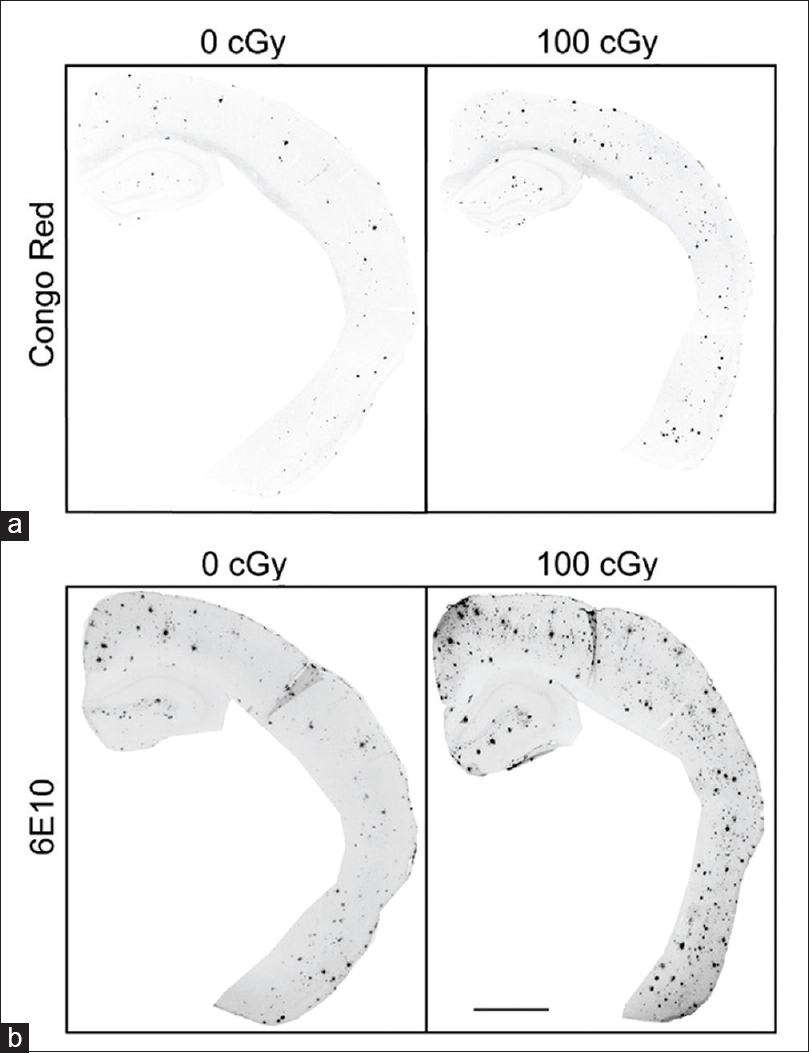
Figure 1
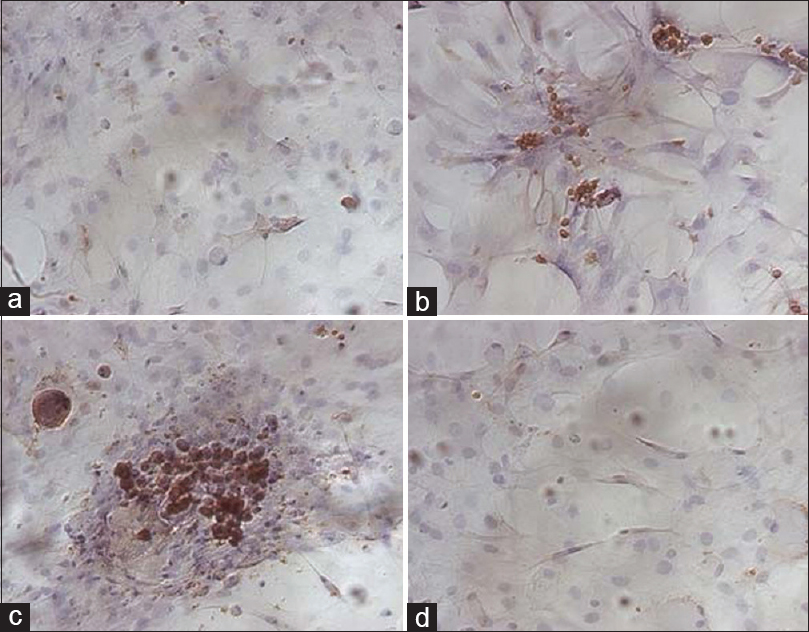
Immunohistochemical staining for Congo red and 6E10 increases after 56Fe particle irradiation. (a, b) Representative images of half male brains stained for Congo red (a) or 6E10 (b) 6 months after 0 Gy or 1 Gy 56Fe particle radiation. Scale bar is 1 mm. Reproduced from Cherry et al. Permission to reproduce open-source figure per Creative Commons 4.0.
While the clinical experience with radiation exposure has provided many important lessons regarding the radiation response of the brain, differences in total doses, dose rate, and importantly, radiation quality have confounded efforts to accurately predict how cosmic radiation exposure might disrupt central nervous system (CNS) functionality. Such prospects are of particular concern to NASA as neurocognitive complications arising from deep space radiation exposure may well compromise astronaut safety, mission success, and post-mission quality of life.[
As alluded to above, differences in radiation quality complicate efforts to estimate space radiation risks due to the different energy deposition patterns that distinguish charged particles found in space from more common terrestrial forms of radiation. Deep space travel subjects astronauts to exposures from potentially large, yet infrequent solar particle events amidst a very low but steady state background of galactic cosmic radiation composed highly energetic charged particles ranging from lighter protons and helium ions to heavier ions such as silicon, titanium, and iron.[
Given this rather dubious backdrop, NASA has invested in research focused on estimating the risks of developing acute (i.e. mission critical) and chronic (i.e. post-mission) CNS deficits. As a result, recent and compelling evidence, based largely on rodent models, has confirmed significant adverse effects of space-relevant fluences of charged particles on cognition,[
A deeper understanding of the underlying mechanisms responsible for space brain provides the most logical route for designing innovative strategies to overcome these looming complications associated with deep space travel. Several lines of evidence suggest that neurotransmission is impaired as a result of multiple pathologies persisting long after exposure. Because behavioral decrements can be predictive of structural alterations, we sought to determine whether regions of the brain interrogated by our cognitive tasks showed signs of damage or change. These studies have now revealed that hippocampal and cortical neurons exhibit significant reductions in dendritic complexity, dendritic spine density, and immature spine morphologies.[
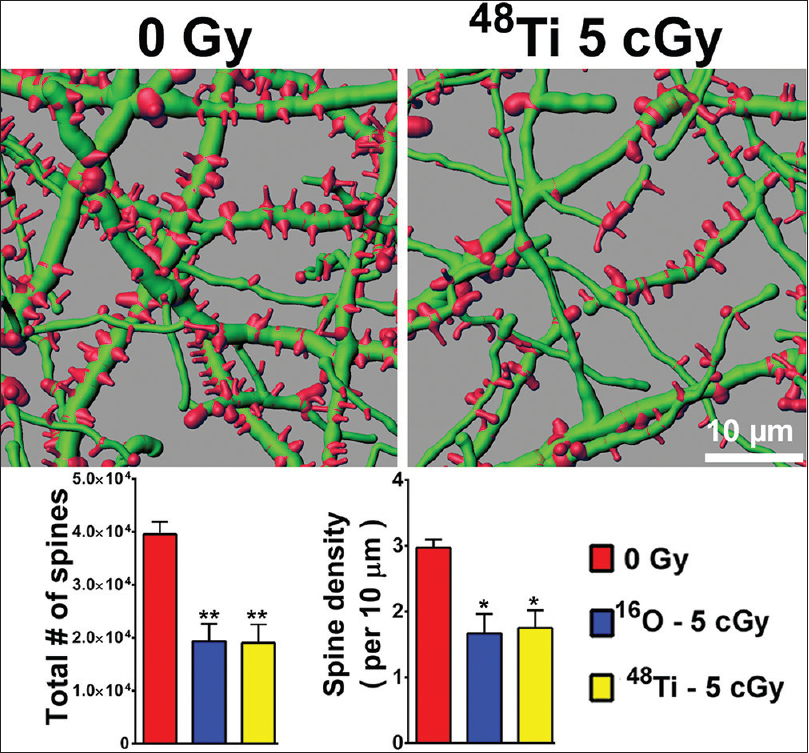
Figure 2
Persistent reductions in dendritic spine density in the entorhinal cortex 6 weeks following cosmic radiation exposure. Representative digital reconstructions of fluorescent dendritic segments (green) in the entorhinal cortex reveal marked reductions in dendritic spines (red) after exposure to titanium ions. Compared to controls, irradiation with either 5 cGy of oxygen or titanium ions reduces total spine numbers (left bar chart) and spine density (right bar chart) significantly (ANOVA). *P < 0.05; **P < 0.01
Importantly, altered neuronal morphology coincides with poor behavioral performance as those animals showing the largest reductions in dendritic spines were found to exhibit the most significant decrements in recognition memory.[
Normal brain function and the burgeoning field of neuroepigenetics have uncovered compelling evidence suggesting that persistent changes in DNA methylation may significantly impact learning and memory. In a recent report, cosmic radiation exposure increased levels of 5-methylcytosine (5mC) and 5-hydroxymethylcytosine (5hmC) in the hippocampus and correlated with persistent impairments in hippocampal and cortical memory.[
In summary, as NASA plans for longer duration manned spaceflight, concerns have surfaced regarding the elevated risks associated with protracted exposure to the highly energetic spectrum of cosmic radiation. Animal models have revealed an unexpected sensitivity of multiple neuronal subtypes in the brain, with corresponding deficits in behavior. While data derived from rodents may be questioned for human relevance, they remain a useful resource for gathering critical information regarding the radiation response of the intact CNS.
Extrapolation of risk models across species will always be fraught with uncertainty but can be reduced through a deeper understanding of the neurobiological mechanisms. Biochemical, molecular, and cellular perturbations involving the release and availability of neurotransmitters, the redistribution and expression of synaptic proteins, the plasticity of neural circuits, and increased neuroinflammation likely converge to compromise neurotransmission at multiple levels. In the end, such factors may prove critical to small teams of astronauts where their capability to properly manage choreographed activities and respond to unexpected situations may be impacted adversely, confounded further by the increased autonomy inherent to prolonged deep space travel.
Anatomical effects on the brain
Subjective reports of blindness by astronauts and cosmonauts have been documented from even the earliest of space flights. Specifically, one astronaut reported a significant decline in visual acuity throughout his mission aboard the international space station (ISS).[
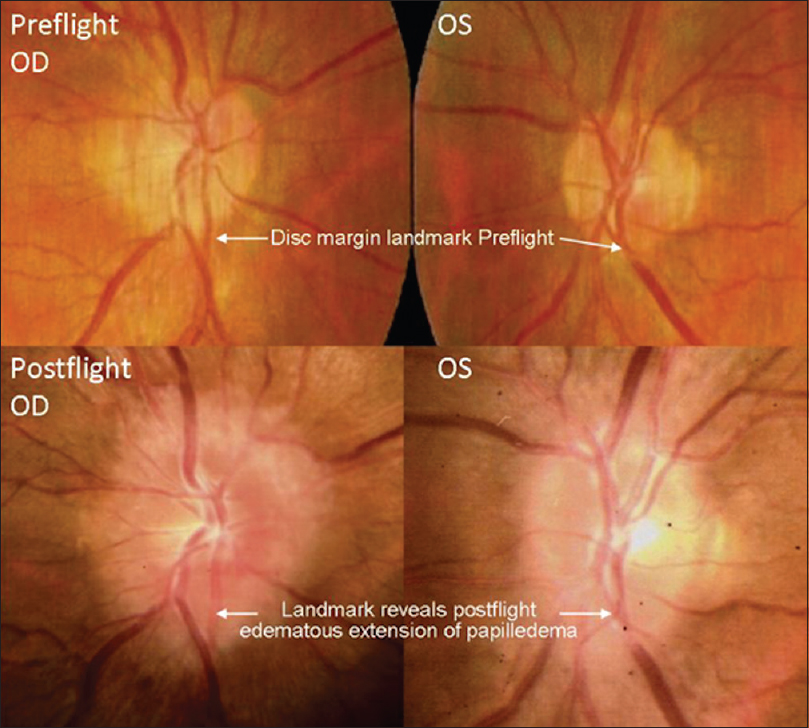
This finding heightened the awareness of ophthalmologic evaluations for astronauts and cosmonauts of future missions. Ophthalmologic examinations were performed on long-duration ISS crew members, which revealed similar ophthalmologic findings. This phenomenon led to the development of a named entity, “Vision Impairment and Intracranial Pressure (VIIP)” by NASA. Specific ophthalmologic findings included hyperopic shifting of up to 1.50 diopters in one or both eyes. Magnetic resonance imaging (MRI) discovered globe flattening. Lumbar punctures were performed in astronauts with evident disc edema, which revealed mildly elevated pressures (range: 21–28.5 cmH2O).[
These symptoms seemed to follow a similar pattern for a different astronaut on a later mission. He had similar ophthalmologic findings, and an MRI was obtained 30 days after landing back on Earth, an MRI was obtained on a different astronaut with newly demonstrated optic disc edema [
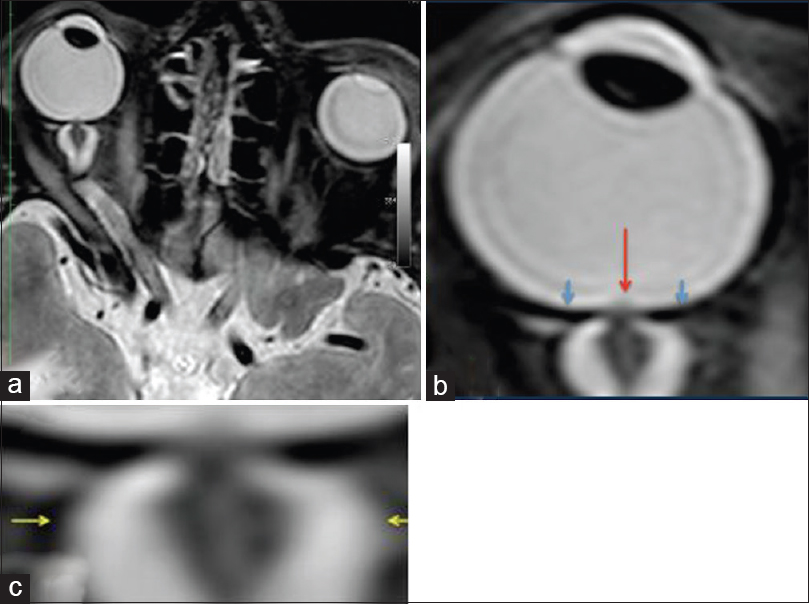
Figure 4
MRI (R + 30 days) of the fourth case of visual changes from long-duration space flight (a). There remains bilateral severe optic sheath dilatation. The right optic sheath diameter measures 10–11 mm (b, c); and the left optic sheath diameter measures 8 mm. These numbers are similar to the R + 3 examination. There is evidence of papilledema on the right eye only. There is residual flattening of the posterior globes. The optic nerve remains thickened bilaterally measuring up to 5 mm on the right and 4 mm on the left. There also remains bilateral tortuosity of the optic nerve sheaths with a kink at the optic nerve sheath approximately 1.1 cm behind the posterior margin of the globe. Red arrow depicts the optic-disc edema, blue arrows show the flattened globe, and the yellow arrows illustrate the distended optic nerve sheath. Reproduced from Mader TH et al.
Following this MRI, a lumbar puncture was obtained, which was 28.5 cmH2O. Cerebrospinal fluid (CSF) analysis was normal for the astronaut.
These phenomena of visual changes associated with anatomic disfigurements of the globe and optic nerve were consistent with the pathophysiology seen in patients with increased intraocular and intracranial pressure.[
Fluid shifting between the intravascular and extravascular spaces have been attributed to physiologic changes in the brain; however, studies remain inconclusive of their effect on the overall electrolyte balance and physiology on the body as a whole. The anticipation and biologic plausibility of decreased intravascular volume due to microgravity was made well-known in early space-flight experiments. However, recent experiments have failed to detect any significant changes in the renin-angiotensin-aldosterone system for short-term missions.[
The vestibular side effects of weightlessness and space have been well-studied. Aside from poor balance and proprioceptive properties of an impaired vestibular system, there are more serious, downstream effects of an improperly functioning vestibular system.[
Oculomotor aberrations have been recorded following prolonged space flight.[
Biological effects on the brain
In addition to the untoward effects of radiation on astronaut's neurobiology, microgravity has been suggested as an instigator in space-related neurologic dysfunction. Gravitational influences have been observed to affect cells at the cytoarchitectural level.[
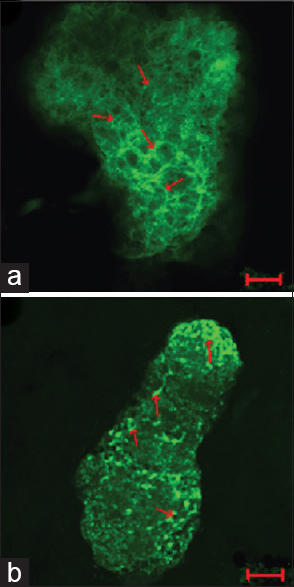
A study performed by He et al.[
Figure 6
The actin cytoskeleton of G2 phase in ground control and test samples in altered gravity for 40 h and subsequent 1 g for about 10 h. The F-actin in Physarum spread on the coverslips was stained with FITC-phalloidin and the actin cytoskeleton was visualized by laser scanning confocal microscopy. Panel (a) showing the control group; panel (b) showing the test samples. Bar: 20 μm. Reproduced from He et al.
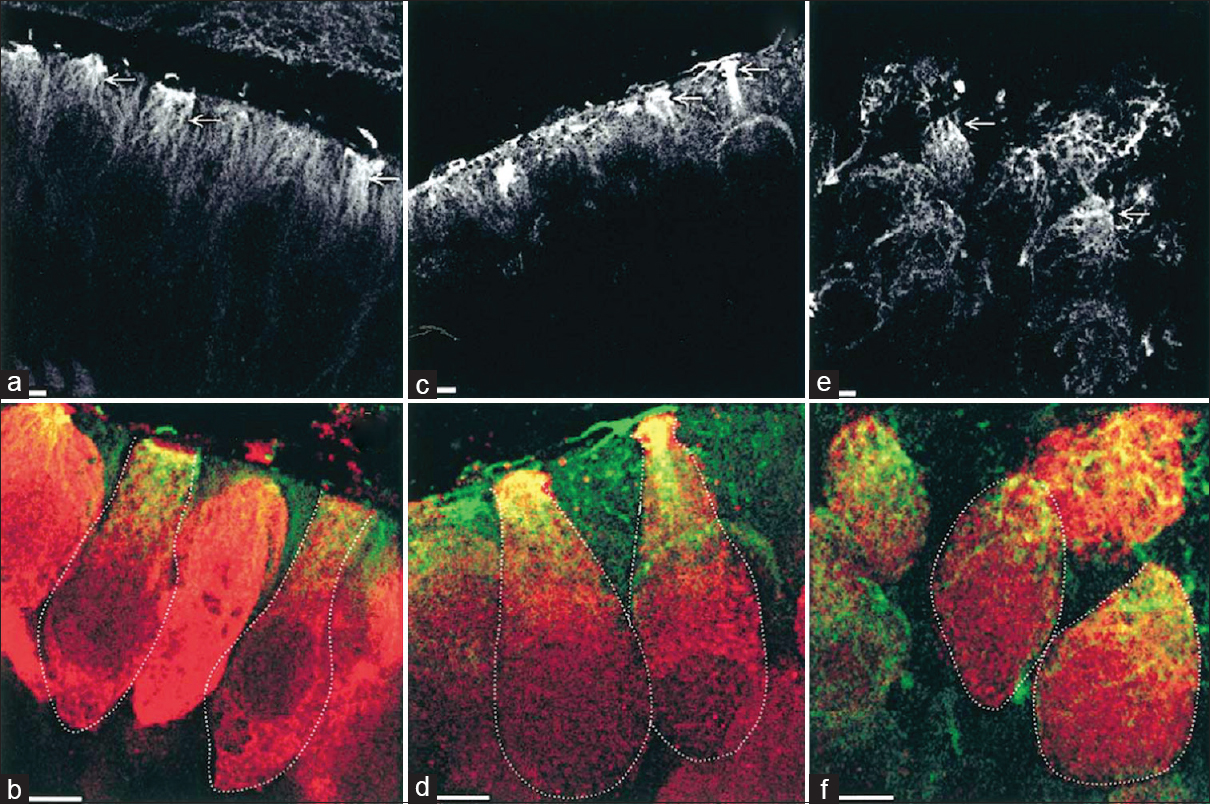
Another experiment by Gaboyard et al.[
Figure 7
Comparison of cytoskeletal architecture (upper frames) and shape (lower frames) of hair cells between ground controls (a and b), C3 in-flight controls (c and d), and C3 weightlessness samples (e and f). α-tubulin staining (a, c, e) shows the organization of microtubules (arrows) in utricular sensory cells. Disorganization of cytoskeletal architecture is seen in weightlessness at higher magnification. (b, d, f), staining for calretinin (red) and a-tubulin (green) show the hair cell shape, demarcated by the dotted lines, and the location of tubulin in the upper part of the cells. Note the differences in hair cell shape between weightlessness samples and controls. Bar = 5 μm. Reproduced from Gaboyard et al.
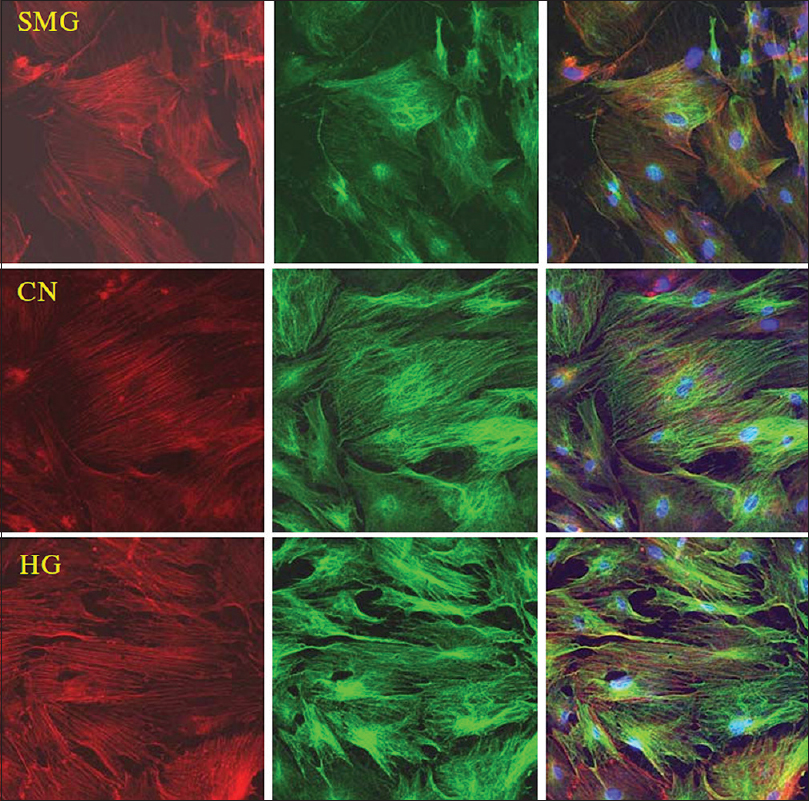
Experiments by Huang et al.[
Figure 8
Effects of HG/SMG on the cytoskeleton of BMSCs. rBMSCs were cultured under HG/SMG conditions for 7 days, and then fixed with 4% paraformaldehyde and stained for microfilaments with Texas red isothiocyanate-conjugated phalloidin (red), microtube cytoskeleton with FITC--conjugated antibody (green) and nucleolus with DAPI (blue). In the SMG group, microfilaments appeared thinner and abnormally distributed, and microtubules appeared diffuse, compared with the control group (CN). In the HG group, the diameters of microfilaments and microtubules appeared to increase. Magnification × 63 oil immersion objective. Reproduced from Huang et al. Permission to reproduce open-source figure per Creative Commons 4.0.
Huang et al. also carried out experiments to determine if gravity played a role in the differentiation of these bone marrow stem cells. Their experiments have proven that gravity plays a functional role in cardiomycyte differentiation [
Figure 9
Immunocytochemistry analysis of cTnT in the cardiomyogenic differentiation of rBMSCs under HG/SMG conditions. (a) rBMSC group was used as a negative control. (b) rBMSCs treated with 5-aza only. (c) CH3 group strongly expressed cTnT. (d) CM3 group had few cTnT positive cells. Magnification ×200. HG increased the expression levels of GATA-4, β-MHC and cTnT in rBMSCs, whereas SMG decreased the expression levels. Reproduced from Huang et al. Permission to reproduce open-source figure per Creative Commons 2.0.
Figure 10
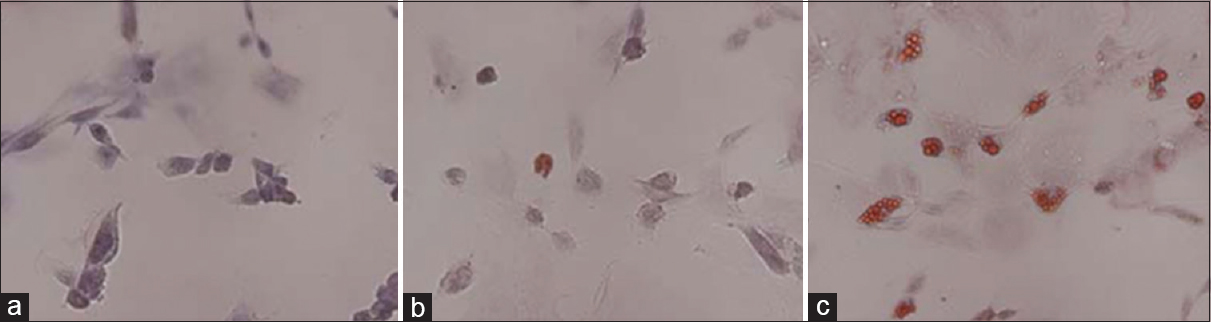
Oil red-O staining to detect the adipogenic differentiation of rBMSCs under HG and SMG conditions. (a) Control group. (b) AH7 showed few oil droplets. (c) AM7 group contained oil droplets in the cells. Magnification ×200. SMG conditions increased the expression of PPARγ2. Reproduced from Huang et al. Permission to reproduce open-source figure per Creative Commons 2.0.
Oxidative stress within the hippocampus is known to be a problem with microgravity.[
Figure 11
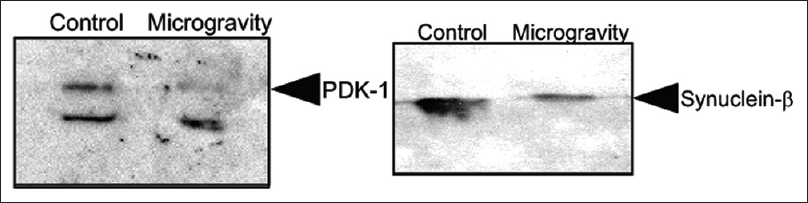
Western blot analysis of Synuclein β in control and in mice kept in simulated microgravity for 7 days. Western blot analysis of pyruvate dehydrogenase (PDK-1) in control and in mice kept in simulated microgravity for 7 days. Reprinted with permission from Sarkar P, Sarkar S, Ramesh V, Hayes BE, Thomas RL, Wilson BL, et al. Proteomic analysis of mice hippocampus in simulated microgravity environment. Journal of proteome research 2006;5(3):548-553. Copyright 2006. American Chemical Society
Specific cells of the hippocampus that control cognitive maps are known as “place cells.” These cells control the ability to translate visuospatial cues into a cognitive map of the subject's environment and surroundings. A study was conducted onboard a Neurolab shuttle mission utilizing mice and a three-dimensional “corner” that represented the floor, ceiling, and wall of a cage.[
Cognitive effects
The known cognitive effects of space travel are mostly a product of radiation exposure as described previously. However, the absence of gravitational exertion can cause radiographic changes in similar neuroanatomic regions, independent of cosmic radiation exposure.
A radiographical analysis of the effects of microgravity was performed by Li et al.,[
Figure 12
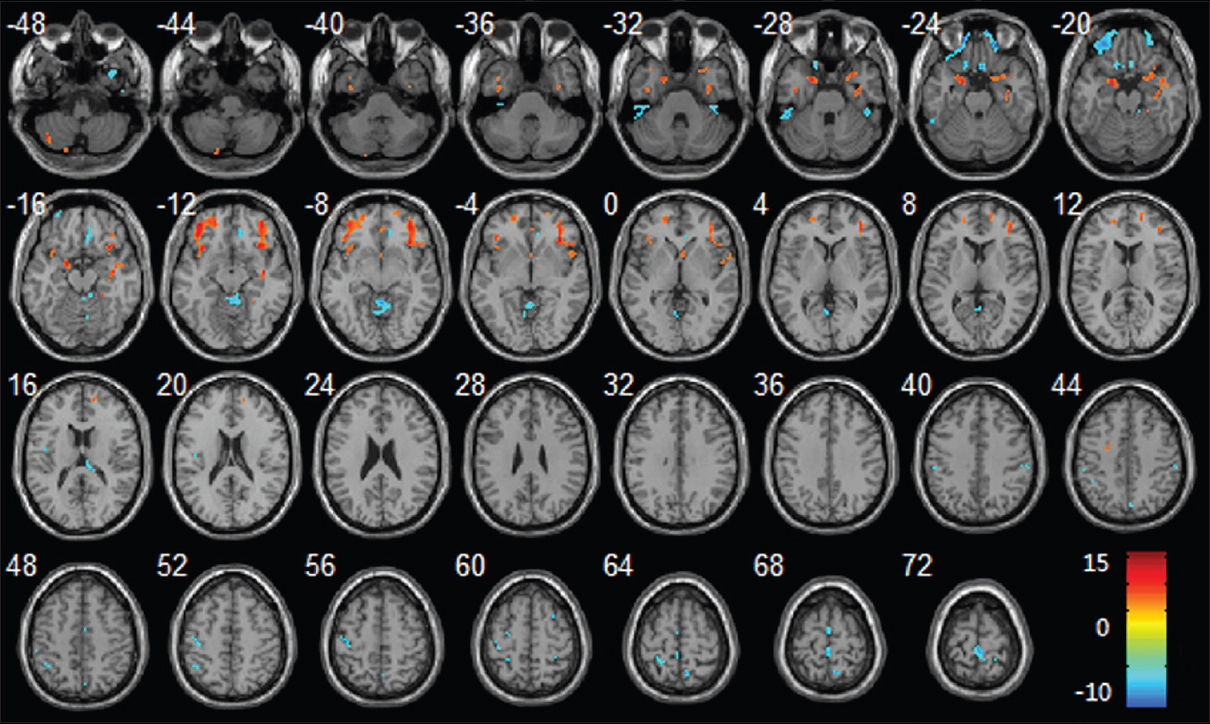
Regional changes of GM volumes after HDBR revealed by voxel-based morphometry. Three-dimensional slices depicting regions showing decreased GM volume (red) in the bilateral frontal lobes, parahippocampal gyrus, insula, right temporal pole, right hippocampus and increased GM volume (blue) in vermis, bilateral paracentral lobule, right precuneus gyrus, left precentral gyrus, left postcentral gyrus overlaid on a T1-weighted MRI anatomical image in the stereotactic space of the Talairach template. Reproduced from Li et al. Permission to reproduce open-source figure per Creative Commons 4.0.
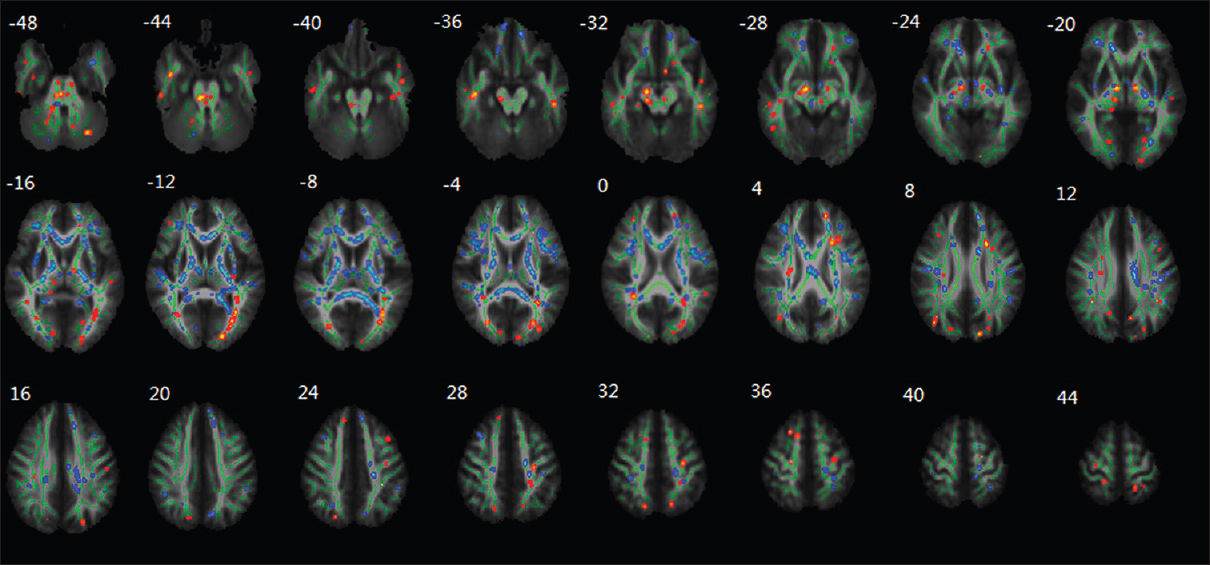
Li et al.'s experiment also discovered aberrations within white matter tracts as well. A decrease in the fractional anisotropy (FA) within the white matter tracts of the frontal lobe, temporal lobe, parietal lobe, occipital lobe, thalamus, brainstem, and cerebellum was observed [
Figure 13
Regional changes of FA values as revealed by TBSS after HDBR. The group's mean FA skeleton (green) was overlaid on the mean FA images. The threshold of mean FA skeleton was set at 0.2; the regions with decreased FA after HDBR are red colored, and the regions with increased FA after HDBR are blue colored. Reproduced from Li et al. Permission to reproduce open-source figure per Creative Commons 4.0.
An alien environment
After landing on Mars, space travellers are at a continued risk of untoward neurologic sequelae. The terrain, atmosphere, and day/night cycle of Mars can interrupt the cognition and performance of space travellers. Aside from the already disrupted circadian function from the rigors of space travel to Mars, specific aspects of Mars itself can attribute to a more accentuated disruption for newly-arrived space travellers.[
Humans will not be the only travellers headed to Mars. The space traveller's gut microbiome will also make the long journey to the red planet, and the effect of space travel has shown to have effects on them as well. The intestinal microbiome is a constant tug-o-war between healthy and harmful bacteria. A small tip in the scales can produce dramatic differences in this equilibrium. As space food have been prepared and packaged beforehand, there is a general sterility of their food for ease of storage and to prevent spoiling. However, helpful bacteria exist in the food we normally eat on Earth, and this reduced intake in healthy bacteria can tip the scales towards a proliferation of harmful bacteria. Moreover, in-vitro spaceflight experiments have shown that Salmonella and Escherichia coli species have been shown to have increased virulence, increased antibiotic resistance, increased resistance to environmental stresses, and increased survival in macrophages compared to ground controls.[
A psychologic condition called the “Earth-out-of-view” phenomenon is a situation where pathologic behavioral changes occur when Earth is no longer in view due to prolonged space missions and journeys.[
Path forward
Countermeasures to offset the deleterious effects of space on neurologic function have been conceptualized. Virtual reality systems have been implemented as a part of astronaut training programs to familiarize astronauts with the challenges of orientation and re-orientation when in space.[
CONCLUSIONS
Maintaining neurologic function on long-term voyages through space exposure is vital for both the health of the astronaut and the security of the mission. Studies and previous observations from returning astronauts and cosmonauts from long missions aboard space stations have demonstrated significant unanticipated concerns. Countermeasures to protect the astronauts from space exposure require further exploration and are vital components in ensuring safe and reliable journey to Mars.
Financial support and sponsorship
This work was supported by NASA Grant NNX15AI22G (CLL).
Conflicts of interest
There are no conflicts of interest.
References
1. Acharya MM, Baddour AA, Kawashita T, Allen BD, Syage AR, Nguyen TH. Epigenetic determinants of space radiation-induced cognitive dysfunction. Sci Rep. 2017. 7: 42885-
2. Alexander D, Gibson C, Hamilton D, Lee S, Mader T, Otto C. Risk of spaceflight-induced intracranial hypertension and vision alterations. Evidence Report, Human Research Program, Human Health Countermeasures Element, version. 2012. 1: 12-
3. Andersen BL, Tewfik HH. Psychological reactions to radiation therapy: Reconsideration of the adaptive aspects of anxiety. J Pers Soc Psychol. 1985. 48: 1024-32
4. Barger LK, Sullivan JP, Vincent AS, Fiedler ER, McKenna LM, Flynn-Evans EE. Learning to live on a Mars day: Fatigue countermeasures during the Phoenix Mars Lander mission. Sleep. 2012. 35: 1423-35
5. Brainard GC, Hanifin JP, Greeson JM, Byrne B, Glickman G, Gerner E. Action spectrum for melatonin regulation in humans: Evidence for a novel circadian photoreceptor. J Neurosci. 2001. 21: 6405-12
6. Bremner JD, Krystal JH, Southwick SM, Charney DS. Functional neuroanatomical correlates of the effects of stress on memory. J Traumatic Stress. 1995. 8: 527-53
7. Britten RA, Davis LK, Johnson AM, Keeney S, Siegel A, Sanford LD. Low (20 cGy) doses of 1 GeV/u (56) Fe--particle radiation lead to a persistent reduction in the spatial learning ability of rats. Radiat Res. 2012. 177: 146-51
8. Britten RA, Davis LK, Jewell JS, Miller VD, Hadley MM, Sanford LD. Exposure to mission relevant doses of 1 GeV/Nucleon (56) Fe particles leads to impairment of attentional set-shifting performance in socially mature rats. Radiat Res. 2014. 182: 292-8
9. Britten RA, Jewell JS, Miller VD, Davis LK, Hadley MM, Wyrobek AJ. Impaired Spatial Memory Performance in Adult Wistar Rats Exposed to Low (5-20 cGy) Doses of 1 GeV/n (56) Fe Particles. Radiat Res. 2016. 185: 332-7
10. Britten RA, Miller VD, Hadley MM, Jewell JS, Macadat E. Performance in hippocampus- and PFC-dependent cognitive domains are not concomitantly impaired in rats exposed to 20cGy of 1GeV/n (56) Fe particles. Life Sci Space Res. 2016. 10: 17-22
11. Chancellor JC, Scott GB, Sutton JP. Space Radiation: The Number One Risk to Astronaut Health beyond Low Earth Orbit. Life (Basel). 2014. 4: 491-510
12. Cherry JD, Liu B, Frost JL, Lemere CA, Williams JP, Olschowka JA, O’Banion MK. Galactic cosmic radiation leads to cognitive impairment and increased abeta plaque accumulation in a mouse model of Alzheimer's disease. PloS One. 2012. 7: e53275-
13. Clement G, Vieville T, Lestienne F, Berthoz A. Modifications of gain asymmetry and beating field of vertical optokinetic nystagmus in microgravity. Neurosci Lett. 1986. 63: 271-4
14. Convertino VA. Interaction of semicircular canal stimulation with carotid baroreceptor reflex control of heart rate. J Vestibular Res. 1998. 8: 43-9
15. Crabbe A, Pycke B, Van Houdt R, Monsieurs P, Nickerson C, Leys N, Cornelis P. Response of Pseudomonas aeruginosa PAO1 to low shear modelled microgravity involves AlgU regulation. Environ Microbiol. 2010. 12: 1545-64
16. Cucinotta F, Alp M, Sulzman F, Wang M. Space radiation risks to the central nervous system. Life Sci Space Res. 2014. 2: 54-69
17. Davis CM, DeCicco-Skinner KL, Hienz RD. Deficits in Sustained Attention and Changes in Dopaminergic Protein Levels following Exposure to Proton Radiation Are Related to Basal Dopaminergic Function. PloS One. 2015. 10: e0144556-
18. Gaboyard S, Blanchard MP, Travo C, Viso M, Sans A, Lehouelleur J. Weightlessness affects cytoskeleton of rat utricular hair cells during maturation in vitro. Neuroreport. 2002. 13: 2139-42
19. Hadley MM, Davis LK, Jewell JS, Miller VD, Britten RA. Exposure to Mission-Relevant Doses of 1 GeV/n (48) Ti Particles Impairs Attentional Set-Shifting Performance in Retired Breeder Rats. Radiat Res. 2016. 185: 13-9
20. Haley GE, Yeiser L, Olsen RH, Davis MJ, Johnson LA, Raber J. Early effects of whole-body (56) Fe irradiation on hippocampal function in C57BL/6J mice. Radiat Res. 2013. 179: 590-6
21. He J, Zhang X, Gao Y, Li S, Sun Y. Effects of altered gravity on the cell cycle, actin cytoskeleton and proteome in Physarum polycephalum. Acta Astronautica. 2008. 63: 915-22
22. Huang Y, Dai ZQ, Ling SK, Zhang HY, Wan YM, Li YH. Gravity, a regulation factor in the differentiation of rat bone marrow mesenchymal stem cells. J Biomed Sci. 2009. 16: 87-
23. Kanas , Nick , Dietrich ManzeySpace psychology and psychiatry. Springer Science & Business Media; 2008. 22:
24. Kaufmann WE, Moser HW. Dendritic anomalies in disorders associated with mental retardation. Cereb Cortex. 2000. 10: 981-91
25. Keith D, El-Husseini A. Excitation Control: Balancing PSD-95 Function at the Synapse. Front Mol Neurosci. 2008. 1: 4-
26. Lee SH, Ledri M, Toth B, Marchionni I, Henstridge CM, Dudok B. Multiple Forms of Endocannabinoid and Endovanilloid Signaling Regulate the Tonic Control of GABA Release. J Neurosci. 2015. 35: 10039-57
27. Lee SH, Dudok B, Parihar VK, Jung KM, Zöldi M, Kang YJ. Neurophysiology of space travel: energetic solar particles cause cell type-specific plasticity of neurotransmission. Brain Structure Funct. 2017. 222: 2345-57
28. Li K, Guo X, Jin Z, Ouyang X, Zeng Y, Feng J. Effect of Simulated Microgravity on Human Brain Gray Matter and White Matter--Evidence from MRI. PloS One. 2015. 10: e0135835-
29. Lonart G, Parris B, Johnson AM, Miles S, Sanford LD, Singletary SJ, Britten RA. Executive function in rats is impaired by low (20 cGy) doses of 1 GeV/u (56) Fe particles. Radiat Res. 2012. 178: 289-94
30. Machida M, Lonart G, Britten RA. Low (60 cGy) doses of (56) Fe HZE-particle radiation lead to a persistent reduction in the glutamatergic readily releasable pool in rat hippocampal synaptosomes. Radiat Res. 2010. 174: 618-23
31. Mader TH, Gibson CR, Pass AF, Kramer LA, Lee AG, Fogarty J. Optic disc edema, globe flattening, choroidal folds, and hyperopic shifts observed in astronauts after long-duration space flight. Ophthalmology. 2011. 118: 2058-69
32. Makale MT, McDonald CR, Hattangadi-Gluth JA, Kesari S. Mechanisms of radiotherapy-associated cognitive disability in patients with brain tumours. Nature reviews. Neurology. 2017. 13: 52-64
33. Markiewicz W, Sablotny R, Keller H, Thomas N, Titov D, Smith P. Optical properties of the Martian aerosols as derived from Imager for Mars Pathfinder midday sky brightness data. J Geophys Res Planets. 1999. 104: 9009-17
34. Meyers CA. Neurocognitive dysfunction in cancer patients. Oncology (Williston Park). 2000. 14: 75-
35. Nelson GA. Space radiation and human exposures, a primer. Radiat Res. 2016. 185: 349-58
36. Oman C. Spatial orientation and navigation in microgravity. Spatial processing in navigation. Imagery Percept. 2007. p. 209-47
37. Parihar VK, Allen BD, Caressi C, Kwok S, Chu E, Tran KK. Cosmic radiation exposure and persistent cognitive dysfunction. Sci Rep. 2016. 6: 34774-
38. Parihar VK, Allen B, Tran KT, Macaraeg T, Chu E, Kwok S. What happens to your brain on the way to Mars. Sci Adv. 2015. 1: e1400256-
39. Parihar VK, Pasha J, Tran KK, Craver BM, Acharya MM, Limoli CL. Persistent changes in neuronal structure and synaptic plasticity caused by proton irradiation. Brain Structure Funct. 2015. 220: 1161-71
40. Preissmann D, Leuba G, Savary C, Vernay A, Kraftsik R, Riederer IM. Increased postsynaptic density protein-95 expression in the frontal cortex of aged cognitively impaired rats. Exp Biol Med (Maywood). 2012. 237: 1331-40
41. Rabin BM, Shukitt-Hale B, Carrihill-Knoll KL, Gomes SM. Comparison of the effects of partial- or whole-body exposures to (1)(6) O particles on cognitive performance in rats. Radiat Res. 2014. 181: 251-7
42. Saei AA, Barzegari A. The microbiome: The forgotten organ of the astronaut's body--probiotics beyond terrestrial limits. Future Microbiol. 2012. 7: 1037-46
43. Sarkar P, Sarkar S, Ramesh V, Hayes BE, Thomas RL, Wilson BL. Proteomic analysis of mice hippocampus in simulated microgravity environment. J Proteome Res. 2006. 5: 548-53
44. Selkoe DJ. Alzheimer's disease is a synaptic failure. Science. 2002. 298: 789-91
45. Smith S, Krauhs J, Leach C. Regulation of body fluid volume and electrolyte concentrations in spaceflight. Adv Space Biol Med. 1997. 6: 123-
46. Soltesz I, Alger BE, Kano M, Lee SH, Lovinger DM, Ohno-Shosaku T. Weeding out bad waves: Towards selective cannabinoid circuit control in epilepsy. Nature reviews. Neuroscience. 2015. 16: 264-77
47. Takashima S, Iida K, Mito T, Arima M. Dendritic and histochemical development and ageing in patients with Down's syndrome. J Intellect Disabil Res. 1994. 38: 265-73
48. Tang Y, Luo D, Rong X, Shi X, Peng Y. Psychological disorders, cognitive dysfunction and quality of life in nasopharyngeal carcinoma patients with radiation-induced brain injury. PloS One. 2012. 7: e36529-
49. van Spronsen M, Hoogenraad CC. Synapse pathology in psychiatric and neurologic disease. Curr Neurol Neurosci Rep. 2010. 10: 207-14
50. Wake H, Moorhouse AJ, Miyamoto A, Nabekura J. Microglia: Actively surveying and shaping neuronal circuit structure and function. Trends Neurosci. 2013. 36: 209-17
51. Wellisch DK, Kaleita TA, Freeman D, Cloughesy T, Goldman J. Predicting major depression in brain tumor patients. Psycho-oncology. 2002. 11: 230-8
52. Whitmire AM, Leveton LB, Barger L, Brainard G, Dinges DF, Klerman E. Risk of performance errors due to sleep loss, circadian desynchronization, fatigue, and work overload. Human health and performance risks of space exploration missions: Evidence reviewed by the NASA Human Research Program. NASA SP-2009-3405. Washington, DC: National Aeronautics and Space Administration; 2009. p.
53. Yates BJ, Kerman IA. Post-spaceflight orthostatic intolerance: Possible relationship to microgravity-induced plasticity in the vestibular system. Brain research. Brain Res Rev. 1998. 28: 73-82
54. Yates BJ, Miller AD. Physiological evidence that the vestibular system participates in autonomic and respiratory control. J Vestibular Res. 1998. 8: 17-25






Tamara
Posted November 25, 2018, 4:10 pm
I am a doctoral student in Neuropsychology with experience in Cognitive Psychology. I would love to read more about this and be kept updated. Prior to graduation, I look forward to completing research in this area especially regarding the “Earth-out-of-view” phenomenon and compensatory strategies to prevent maladaptive responses in astronauts on the Martian surface. Thank you for this read.