- Department of Neurosurgery, University Hospital Leuven, Belgium
- Department of Neurosurgery, Jessa Hospital Hasselt, Belgium
- Department of Radiology, Jessa Hospital Hasselt, Belgium
Correspondence Address:
Sven Bamps
Department of Neurosurgery, University Hospital Leuven, Belgium
DOI:10.4103/sni.sni_329_17
Copyright: © 2017 Surgical Neurology International This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Sven Bamps, Eric Put, Termote Bruno, Frank Van Calenbergh. Spinal cord herniation after brachial plexus injury. 27-Dec-2017;8:305
How to cite this URL: Sven Bamps, Eric Put, Termote Bruno, Frank Van Calenbergh. Spinal cord herniation after brachial plexus injury. 27-Dec-2017;8:305. Available from: http://surgicalneurologyint.com/surgicalint-articles/spinal-cord-herniation-after-brachial-plexus-injury/
Abstract
Background:Spinal cord herniation (SCH) is an uncommon cause of myelopathy. Documented trauma is a rare cause, and most cases are idiopathic. One special type of trauma that may lead to SCH is a brachial plexus injury. We report a case of SCH with delayed neurological symptoms after a brachial plexus injury. We reviewed the literature and illustrated the closing technique as described by Batzdorf.
Case Description:Following a motor vehicle accident, a 27-year-old male sustained a brachial plexus injury and multiple left-sided nerve root avulsions (C6, C7, and C8) resulting into a full paralysis of the left arm. There was also a loss of pain and temperature sensation on the right side of the body. He underwent reconstructive surgery without any functional improvement. After 6 to 7 years his condition worsened. Magnetic resonance imaging revealed a left-sided SCH at the level of C7. He underwent a C6-C7 laminectomy which revealed a pseudomeningocele at C6-C7 accompanied by focal SCH at the location of the C7 root. The SCH was reduced intradurally and the dural defect of the meningocele was covered with a Neuropatch membrane wrapped around the spinal cord (between the spinal cord and the dura) according to the technique described by Batzdorf. Postoperatively, the neurological symptoms improved.
Conclusion:SCH should be surgically repaired utilizing the technique described by Batzdorf if further neurological deficits develop.
Keywords: Batzdorf surgical repair, myelopathy, spinal cord herniation
INTRODUCTION
Spinal cord herniation (SCH) is a rare cause of myelopathy. The diagnosis and treatment can be challenging, and most patients have a delay in diagnosis as the progression of symptoms is often insidious. Etiologies of SCH rarely include trauma as most are idiopathic. We report a case in which a patient sustained delayed onset of a progressive myelopathy attributed to a SCH occurring years after a brachial plexus injury involving avulsion of the C6, C7, C8 nerve roots. Here, we reviewed the literature and utilized the closure technique described by Batzdorf.
CASE REPORT
History
A 27-year-old male was involved in a traffic accident that resulted in a left-sided brachial plexus injury resulting in avulsion of the C6, C7, and C8 nerve roots. He sustained a full paralysis of the left arm accompanied by a loss of pain and temperature sensation on the right side of the body. He underwent reconstructive surgery without any functional improvement. After 6 to 7 years he developed progressive worsening myelopathy (e.g., increased stiffness/gait difficulty, atrophy) in the left leg.
Neurological examination revealed a complete, paralysis of the C6-, C7- and C8-innervated muscle groups of the left arm with areflexia and sensory loss. In the left leg, there was new atrophy of the quadriceps and gastrocnemius muscle with hyperreflexia and a diffuse mild paresis with a foot drop. There was additional sensory loss on the right side of the body starting at the T4-dermatome. The gait was spastic on the left side.
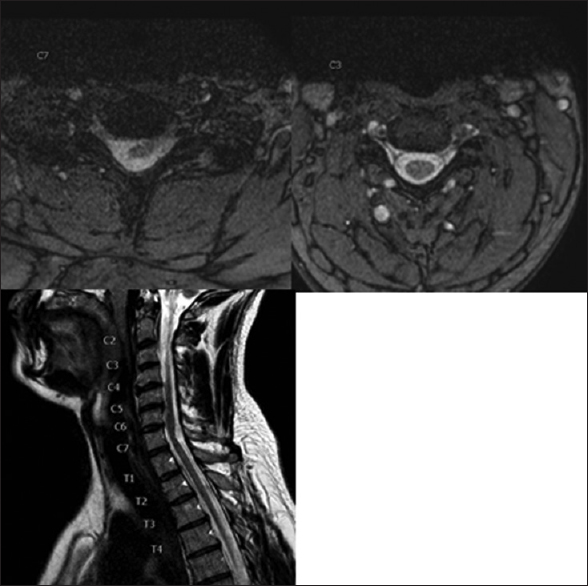
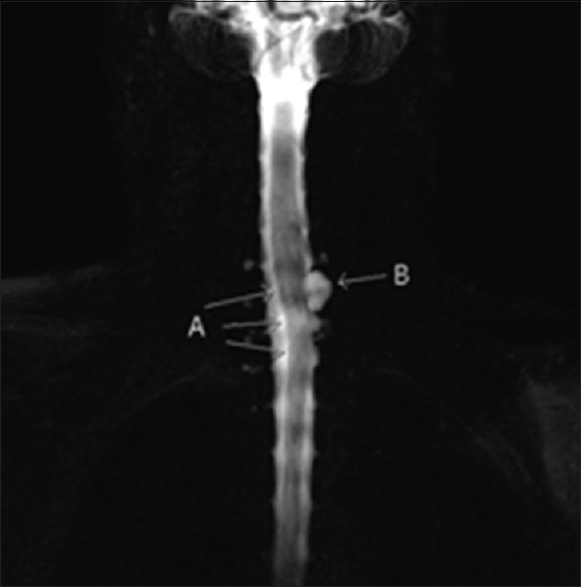
T2-weighted magnetic resonance imaging (MRI) (1.5 T) revealed left-sided SCH at the C7 root level; this was characterized by anterior left-sided displacement of the spinal cord towards the C8 root foramen with stretching and flattening of the cord [
Figure 1
Axial T2-weighted images. Right image showing normal position of the cervical cord at level C3. Left image at level C7 showing left-sided cord herniation: anterior and left-sided displacement of the cord towards the foramen of root C8 resulting in the stretching and flattening of the cord. Sagittal T2-weighted left-sided off midline image. Anterior displacement of the cord against the posterior aspect of level C4 to C6 and posterior indentation on/anterior angulation of the cord on level C7
Operation
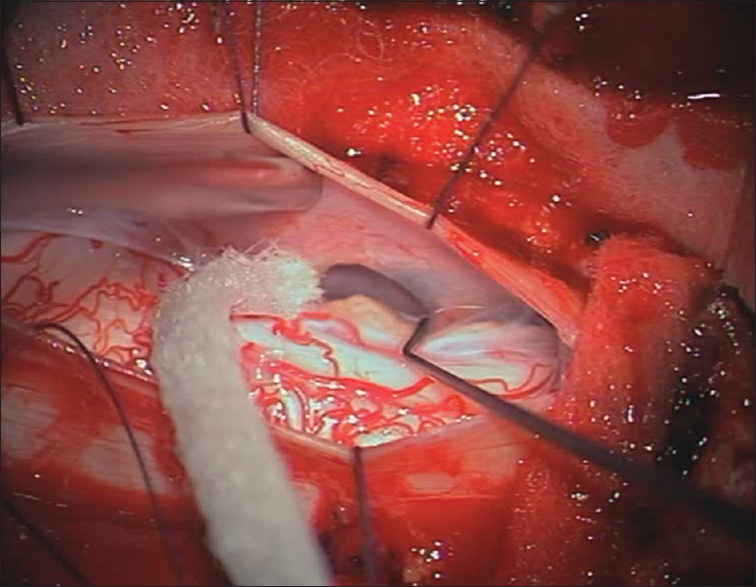
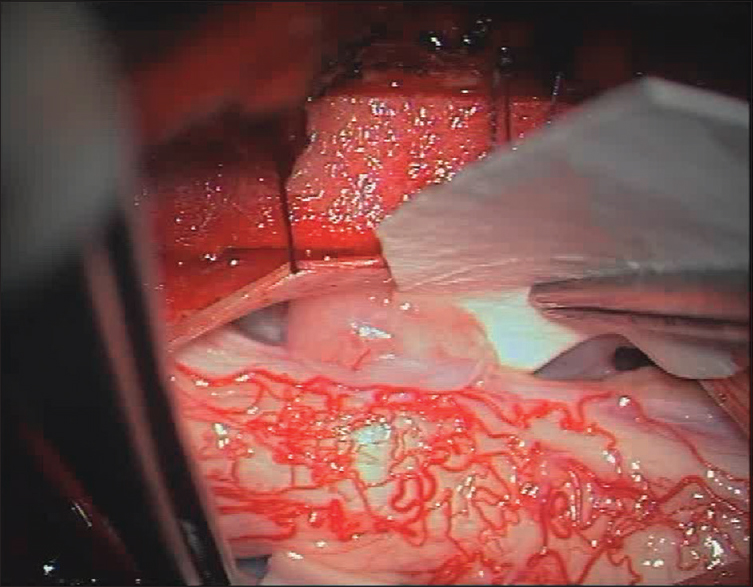
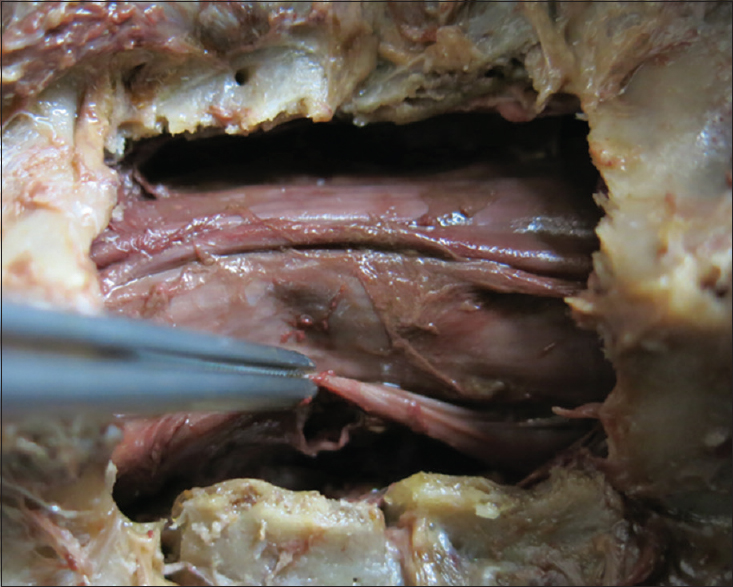
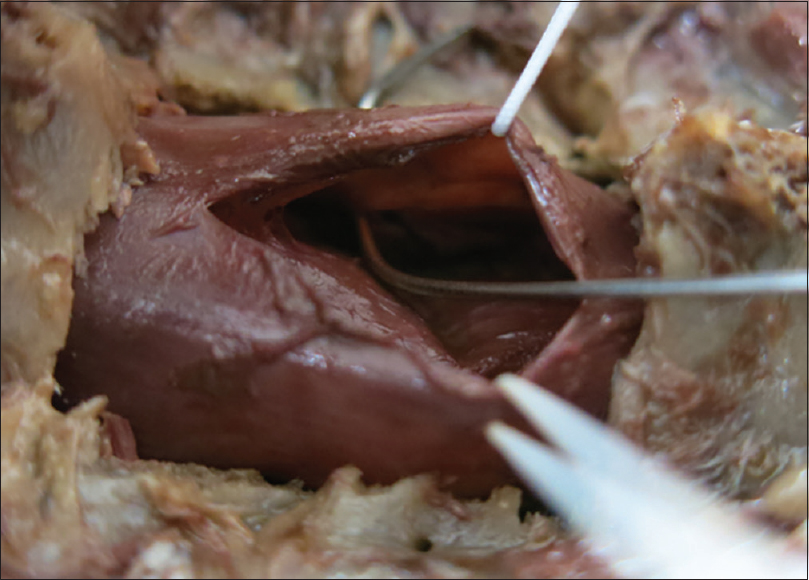
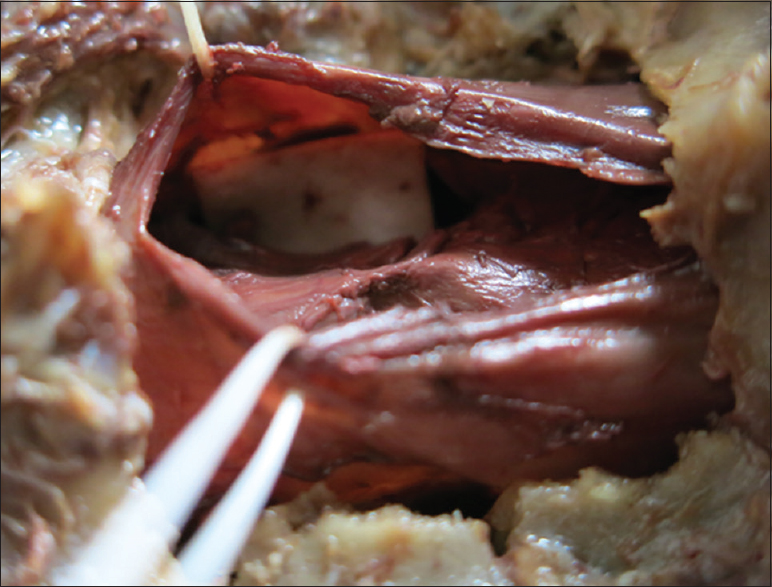
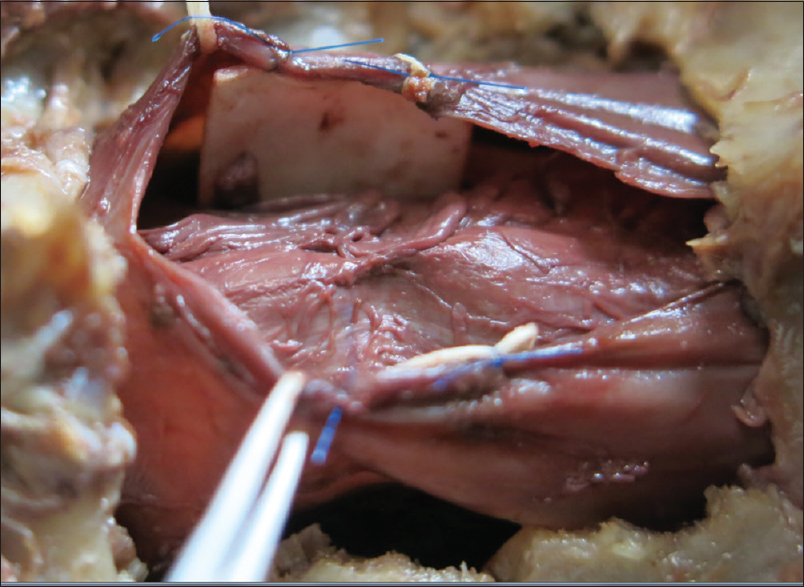
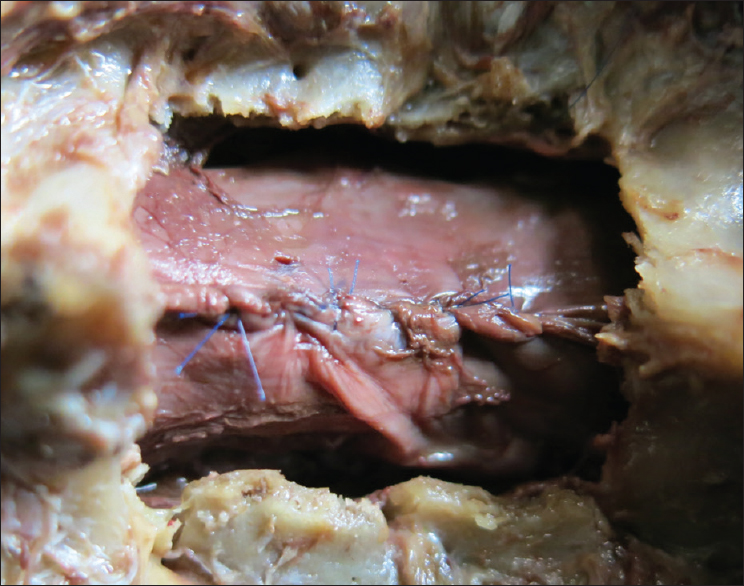
The patient underwent a C6-C7 laminectomy which revealed a pseudomeningocele at the C6-C7 on the left side. Upon opening the dura in the midline, the spinal cord was covered with a thickened arachnoid. It was herniated into the pseudomeningocele at the C7 root level and there were accompanying adhesions. The herniated spinal cord was reduced intradurally [
Postoperative course
The postoperative course was uneventful and the neurological symptoms improved clearly; there was an increase in strength in the left leg and he was able to walk more normally at 6 and 12 weeks of follow-up.
DISCUSSION
Spinal trauma can cause a dural tear and persistent dural rent with a ruptured arachnoid leading to extravasation of cerebrospinal fluid creating a pseudomeningocele.[
A long period is needed for a pseudomeningocele to become symptomatic. If a pseudomeningocele extends along the spinal canal causing compression or adhesions onto the spinal cord, delayed neurological symptoms may occur. In this case, increased myelopathy was noted along with greater corticospinal and spinothalamic tract resulting in an ipsilateral motor deficit and a contralateral sensory loss for pain and temperature [Figures
Only a few cases of SCH after a brachial plexus avulsion injury which resulted in a herniation of the spinal cord into a pseudomeningocele have been reported. In those cases, myelopathy showed an early improvement after reduction of the SCH, with dural repair.[
CONCLUSION
SCH should be surgically repaired utilizing the technique described by Batzdorf [Figures
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient has given her consent for her images and other clinical information to be reported in the journal. The patient understands that name and initial will not be published and due efforts will be made to conceal identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Batzdof U, Langston T. Idiopathic thoracic spinal cord herniation: Report of 10 patients and description of surgical approach. J Spinal Disord Tech. 2012. 25: 157-
2. Borges LF, Zervas NT, Lehrich JR. Idiopathic spinal cord herniation: A treatable cause of the Brown-Sequard syndrome: Case report. Neurosurgery. 1995. 36: 1028-
3. Burres KP, Conley FK. Progressive neurological dysfunction secondary to postoperative cervical pseudomeningocele in a C-4 quadriplegic: Case report. J Neurosurg. 1978. 48: 289-
4. Hosono N, Yonenobu K, Ono K. Postoperative cervical pseudomeningocele with herniation of the spinal cord. Spine. 1995. 20: 2147-
5. Sklar EM, Quencer RM, Green BA. Posttraumatic spinal pseudomeningocele: MR and clinical features. AJNR Am J Neuroradiol. 1990. 11: 1184-
6. Yokota H, Yokoyama K, Noguchi H, Uchiyama Y. Spinal cord herniation into associated pseudomingocoele after brachial plexus avulsion injury: Case report. Neurosurgery. 2007. 60: 205-