- Department of Neurosurgery, Clinical Research Institute, National Hospital Organization, Kyushu Medical Center, Fukuoka, Japan
- Department of Pathology, Clinical Research Institute, National Hospital Organization, Kyushu Medical Center, Fukuoka, Japan
Correspondence Address:
Yutaka Fujioka
Department of Pathology, Clinical Research Institute, National Hospital Organization, Kyushu Medical Center, Fukuoka, Japan
DOI:10.4103/sni.sni_318_18
Copyright: © 2019 Surgical Neurology International This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.How to cite this article: Yutaka Fujioka, Toshiyuki Amano, Akira Nakamizo, Satoshi Matsuo, Shigeto Kawauchi. A case of metastatic brain tumor mimicking an expanding thalamic hematoma. 15-Jan-2019;10:3
How to cite this URL: Yutaka Fujioka, Toshiyuki Amano, Akira Nakamizo, Satoshi Matsuo, Shigeto Kawauchi. A case of metastatic brain tumor mimicking an expanding thalamic hematoma. 15-Jan-2019;10:3. Available from: http://surgicalneurologyint.com/surgicalint-articles/9191/
Abstract
Background:Brain tumor are a major etiology of secondary intracranial hemorrhage (ICH) because ICH in patients with cancer often occurs from an intratumoral hemorrhage. However, it is sometimes difficult to detect a tumor when it is tiny and buried, especially during initial examination.
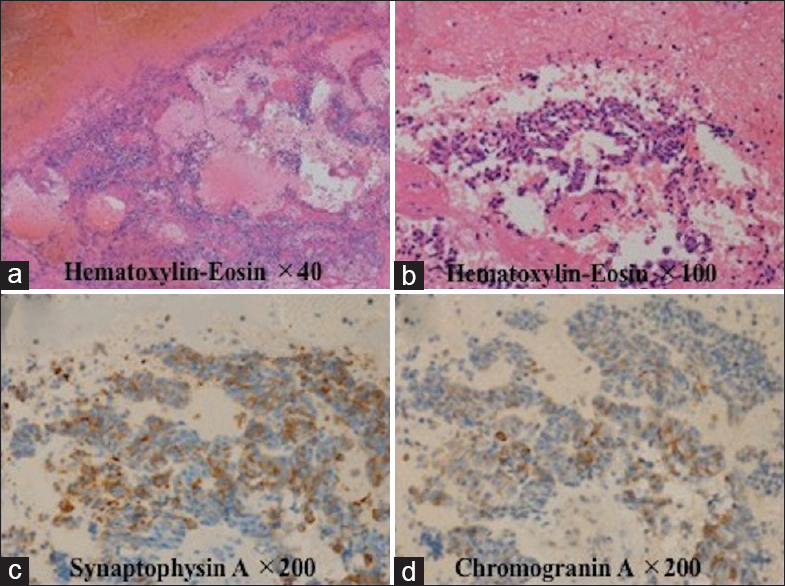
Case Description:A 65-year-old woman who was diagnosed with pulmonary small cell carcinoma 6 months previously developed sudden-onset consciousness disturbance and left hemiparesis. Head computed tomography (CT) showed a round, high-density lesion with a diameter of 31 mm in the right thalamus. There was no enhancement with administration of contrast agent. Five days later, CT revealed significant progression of the hematoma in the thalamus with perifocal edema. She underwent total removal of the hematoma. Histopathological examination revealed a tiny cluster of metastatic cancer tissue within the hematoma.
Conclusions:When cerebral hemorrhage occurs in a cancer patient, we must consider the possibility of hemorrhage due to a brain metastasis.
Keywords: Expanding hematoma, intratumoral hemorrhage, metastatic brain tumor, small-cell lung cancer, thalamic hemorrhage
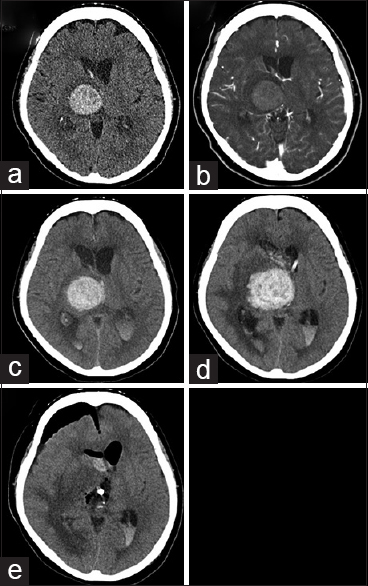
A 65-year-old woman was diagnosed with pulmonary small-cell carcinoma 6 months previously developed sudden-onset consciousness disturbance and left hemiparesis. Her clinical examination revealed a Glasgow Coma Scale (GCS) score of 10 (E3, V3, M4) and severe motor dysfunction (manual muscle test, 2/5). Head computed tomography (CT) showed a 31-mm diameter round, high-density lesion in the right thalamus [
Figure 1
Radiological examination: (a) computed tomography (CT) showing a high-density area in the right thalamus at admission; (b) CT showing no enhancement of the hematoma following contrast agent administration at admission; (c) CT showing acute hydrocephalus with mild progression of the hematoma 3 h after admission; (d) CT showing significant hematoma progression 5 days after hospitalization; and (e) postoperative CT showing total removal of the right thalamic hemorrhage 6 days after hospitalization
External ventricular drainage was performed after 3 h because acute hydrocephalus progressed, and her consciousness deteriorated [
Considering the prognosis of the primary tumor, radiotherapy was not administered at the family's request; 30 days after hospitalization, she was transferred to receive palliative medical care. Written informed consent was obtained from the patient and her family.
Brain metastases occur in the gray/white matter region of the cerebral hemisphere. However, metastasis of cancer cells to the thalamus is uncommon (2.2%).[
The etiology of intracerebral hemorrhage (ICH) in cancer patients is heterogeneous. The largest clinical series, including 208 cancer patients who developed ICH or subarachnoid hemorrhage, showed that 61% (n = 127) of hemorrhages were caused by intratumoral hemorrhage, 46% (n = 95) by coagulopathy, 33% (n = 69) by multifactorial causes, and 21% (n = 44) by intratumoral hemorrhage and coagulopathy.[
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
We would like to thank Editage (www.editage.jp) for English language editing.
References
1. . Committee of Brain Tumor Registry of Japan. Report of brain tumor registry of Japan (2001-2004). Neurologia Medico-chirurgica. 2014. 54: 9-102
2. Kondziolka D, Berstein M, Resch L, Tator CH, Fleming JFR, Vanderlind RG. Significance of hemorrhage into brain tumors: Clinicopathological study. J Neurosurg. 1987. 67: 852-7
3. Velander AJ, DeAngelis LM, Navi BB. Intracranial hemorrhage in patients with cancer. Curr Atheroscler Rep. 2012. 14: 373-81
4. Zakaria R, Kumar D, Bhojak M, Radon M, Walker C, Jenkinson M. The role of magnetic resonance imaging in the management of brain metastases: Diagnosis to prognosis. Cancer Imaging. 2014. 14: 8-