- Departments of Neurosurgery, Kindai University Faculty of Medicine, 377-2 Ohno-Higashi, Osaka-Sayama, Osaka, Japan
- Departments of Neurosurgery, Nara Hospital, Kindai University Faculty of Medicine, 1248-1 Otodacho, Ikoma, Nara, Japan.
Correspondence Address:
Kiyoshi Tsuji
Departments of Neurosurgery, Kindai University Faculty of Medicine, 377-2 Ohno-Higashi, Osaka-Sayama, Osaka, Japan
DOI:10.25259/SNI-78-2019
Copyright: © 2019 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Kiyoshi Tsuji, Akira Watanabe, Nobuhiro Nakagawa, Amami Kato. A case of unilateral vertebral artery dissection progressing in a short time period to bilateral vertebral artery dissection. 28-Jun-2019;10:126
How to cite this URL: Kiyoshi Tsuji, Akira Watanabe, Nobuhiro Nakagawa, Amami Kato. A case of unilateral vertebral artery dissection progressing in a short time period to bilateral vertebral artery dissection. 28-Jun-2019;10:126. Available from: http://surgicalneurologyint.com/surgicalint-articles/9438/
Abstract
Background: Vertebral artery dissection (VAD) is an important cause of stroke in young and middle- aged people. Bilateral occurrence of VAD is generally considered rare, but the number of reports of bilateral VAD has been increasing in recent years. In this paper, we report a case of de novo VAD on the contralateral side presenting with subarachnoid hemorrhage in the acute stage of cerebral infarction due to unilateral VAD.
Case Description: A 52-year-old man developed sudden-onset left occipital headache, dizziness, dysphagia, and right-sided hemiparesthesia and was admitted to our hospital. Head magnetic resonance imaging on admission showed a left lateral medullary infarction due to the left VAD. At this point, the right vertebral artery was normal. However, on day 9 after onset, he suddenly presented with subarachnoid hemorrhage due to the right VAD. Emergency endovascular treatment was performed for the dissecting aneurysm of the right vertebral artery. The patient’s condition improved gradually after the procedure, and he was discharged with a modified Rankin Scale score of 1.
Conclusion: Bilateral occurrence of VAD may be more common than previously believed. Even in cases of unilateral VAD, we need to pay attention to the occurrence of de novo VAD on the contralateral side.
Keywords: Bilateral vertebral artery dissection, de novo vertebral artery dissection, Stent-assisted coil embolization, Subarachnoid hemorrhage, Vertebral artery dissecting aneurysm
INTRODUCTION
Vertebral artery dissection (VAD; referring to intracranial VAD in this paper) is an important cause of stroke in young and middle-aged people.[
CASE DESCRIPTION
A 52-year-old man with no particular medical history developed sudden-onset left occipital headache, dizziness, dysphagia, and right-sided hemiparesthesia and was transported to another hospital. Head magnetic resonance imaging (MRI) revealed a left lateral medullary infarction due to the left VAD, and he was admitted to that hospital. Conservative treatment was performed, and the patient had an uneventful course, but at his and his family’s request, he was transferred to our hospital 4 days after onset.
On admission to our hospital, head MRI was performed again. The MRI findings were the same as those observed at the other hospital; that is, a left lateral medullary infarction due to the left VAD was observed, and there were no abnormal findings in the right vertebral artery [
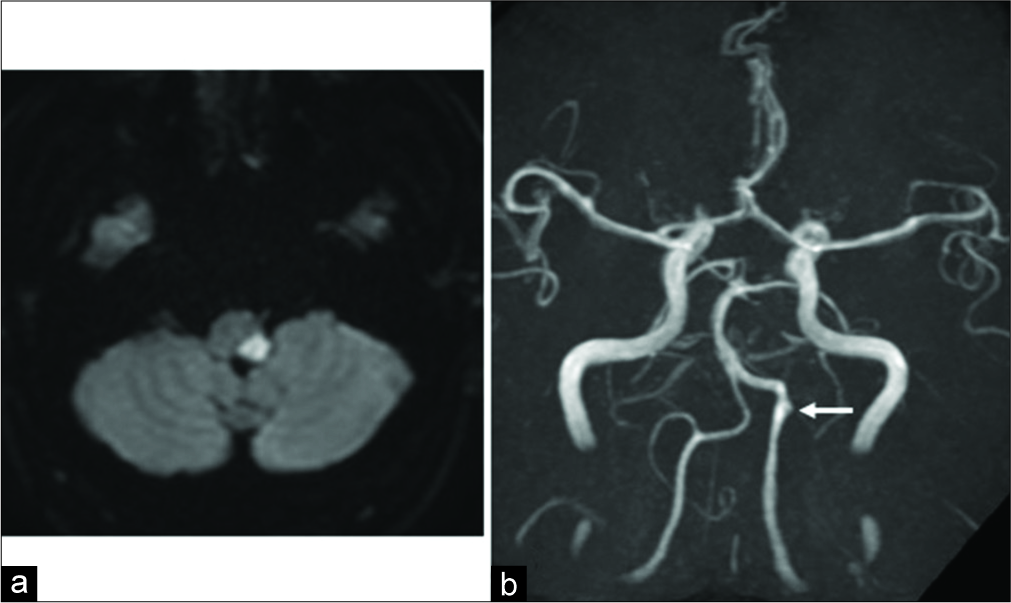
Figure 1:
(a) Magnetic resonance diffusion-weighted imaging shows acute cerebral infarction in the left lateral medulla. (b) Magnetic resonance angiography shows irregularities of the vessel wall (arrow) suggestive of dissection in the left vertebral artery. No abnormal findings are observed in the right vertebral artery.
On day 9 after onset, the patient developed sudden disturbance of consciousness and was found lying on the side of his bed by a nurse. Head computed tomography (CT) showed SAH, mainly in the posterior cranial fossa [
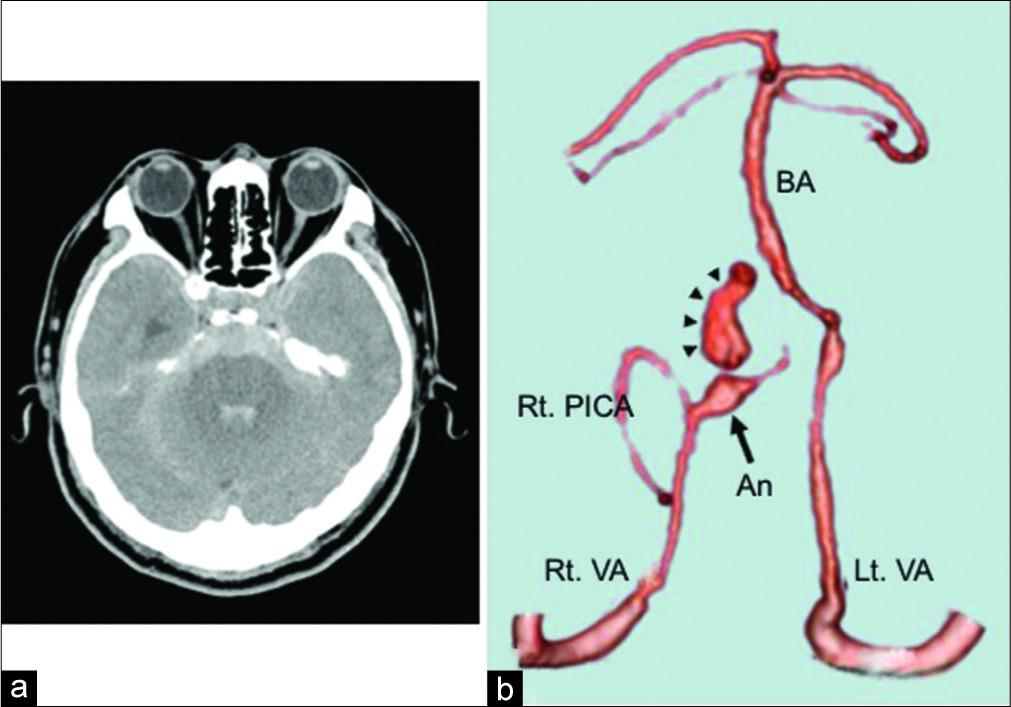
Figure 2:
(a) Plain computed tomography (CT) shows diffuse subarachnoid hemorrhage, mainly in the posterior cranial fossa. (b) Three-dimensional CT angiography shows a dissecting aneurysm (arrow) with active extravasation of contrast agent (arrowhead) in the right vertebral artery distal to the posterior inferior cerebellar artery. An: Aneurysm; BA: Basilar artery; Lt. VA: Left vertebral artery; Rt. PICA: Right posterior inferior cerebellar artery; Rt. VA: Right vertebral artery.
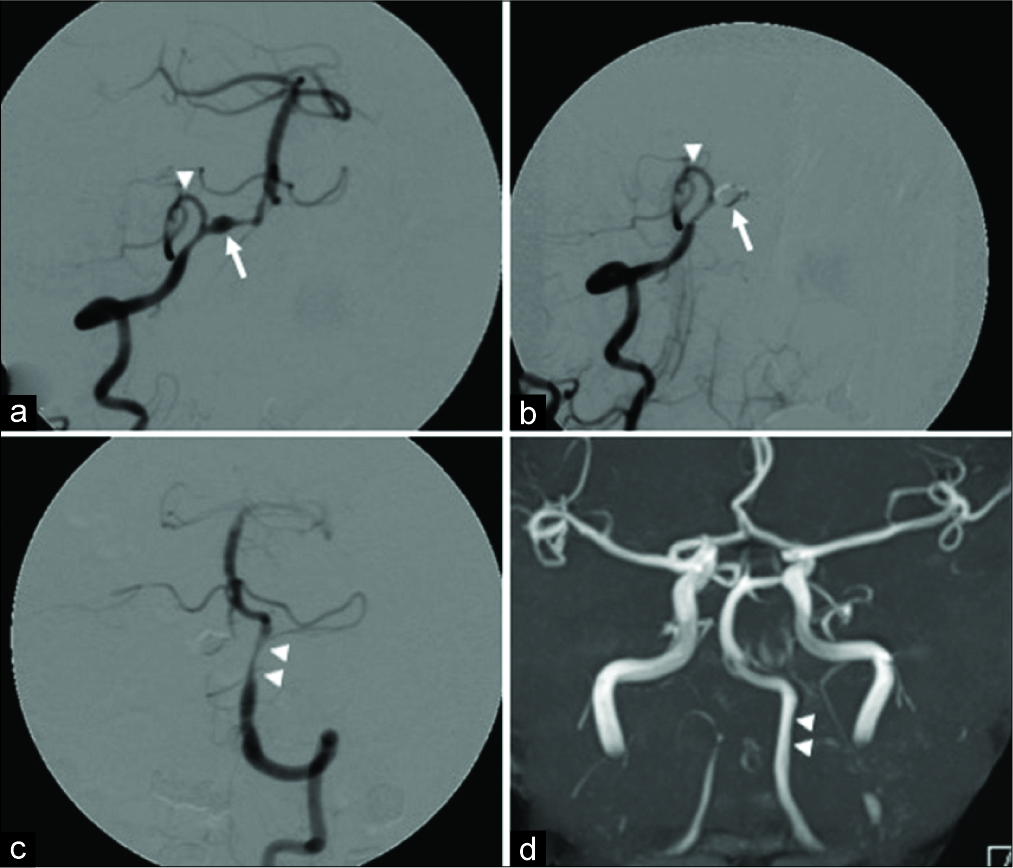
Figure 3:
(a) The right anterior oblique view of the right vertebral artery angiography. A dissecting aneurysm (arrow) is observed in the right vertebral artery distal to the posterior inferior cerebellar artery. The arrowhead indicates the right posterior inferior cerebellar artery. (b) The right anterior oblique view of the right vertebral artery angiography after internal trapping. The dissecting aneurysm of the right vertebral artery is completely embolized by coils (arrow), and blood flow of the right posterior inferior cerebellar artery is preserved (arrowhead). (c) Anteroposterior view of the left vertebral artery angiography after internal trapping. The moderate stenosis of the left vertebral artery due to dissection is observed (arrowhead), but the basilar artery is visualized in antegrade with no delay of blood flow. (d) Magnetic resonance angiography approximately 1 month after onset. The stenosis of the left vertebral artery due to dissection has resolved (arrowhead).
DISCUSSION
The cerebral arterial walls consist of four layers: From the inside, the tunica intima, internal elastic lamina, tunica media, and tunica adventitia. In cerebral arteries, which are typical muscular arteries, the elastic fibers that determine the strength of the vessel wall are sparse in the tunica media and abundant in the internal elastic lamina.[
In the present case, de novo VAD on the contralateral side occurred in the acute stage of cerebral infarction due to unilateral VAD, and, as a result, the patient presented with SAH. Why did this kind of surprising phenomenon occur? There is an interesting autopsy study that answers this question. Ro et al.[
In the diagnosis of VAD, MRA is widely used. However, in some cases, ordinary MRA alone may not be able to detect VAD. Basiparallel anatomic scanning is a method designed to visualize the surface appearance of the vertebrobasilar artery within the cistern. The combination of MRA and basiparallel anatomic scanning enables a more accurate diagnosis of VAD.[
With recent advances in device technologies, endovascular treatment has become the first-line treatment for vertebral artery dissecting aneurysms.[
CONCLUSION
Bilateral vertebral arteries of a patient with VAD may be vulnerable. Therefore, when managing a patient with VAD, we need to carefully monitor not only the unilateral vertebral artery but also the contralateral vertebral artery. In the treatment of vertebral artery dissecting aneurysms, stent-assisted coil embolization with preservation of blood flow of parent artery would be the ideal treatment method.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patient has given his consent for his images and other clinical information to be reported in the journal. The patient understands that his name and initials will not be published, and due efforts will be made to conceal his identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
None.
Conflicts of interest
There are no conflicts of interest.
References
1. Inui Y, Oiwa Y, Terada T, Nakakita K, Kamei I, Hayashi S. De novo vertebral artery dissecting aneurysm after contralateral vertebral artery occlusion two case reports. Neurol Med Chir (Tokyo). 2006. 46: 32-6
2. Ishikawa T, Yamaguchi K, Anami H, Ishiguro T, Matsuoka G, Kawamata T. Stent-assisted coil embolisation for bilateral vertebral artery dissecting aneurysms presenting with subarachnoid haemorrhage. Neuroradiol J. 2016. 29: 473-8
3. Katsuno M, Mizunari T, Kobayashi S, Takahashi H, Teramoto A. Rupture of a vertebral artery dissecting aneurysm developing immediately after trapping of a dissecting aneurysm on the contralateral vertebral artery: Case report. Neurol Med Chir (Tokyo). 2009. 49: 468-70
4. Kidani N, Sugiu K, Hishikawa T, Hiramatsu M, Haruma J, Nishihiro S. De novo vertebral artery dissecting aneurysm after internal trapping of the contralateral vertebral artery. Acta Neurochir (Wien). 2017. 159: 1329-33
5. Kono K, Shintani A, Fujimoto T, Terada T. Stent-assisted coil embolization and computational fluid dynamics simulations of bilateral vertebral artery dissecting aneurysms presenting with subarachnoid hemorrhage: Case report. Neurosurgery. 2012. 71: E1192-200
6. Metso TM, Metso AJ, Helenius J, Haapaniemi E, Salonen O, Porras M. Prognosis and safety of anticoagulation in intracranial artery dissections in adults. Stroke. 2007. 38: 1837-42
7. Mizutani T, Aruga T, Kirino T, Miki Y, Saito I, Tsuchida T. Recurrent subarachnoid hemorrhage from untreated ruptured vertebrobasilar dissecting aneurysms. Neurosurgery. 1995. 36: 905-11
8. Mizutani T, Kojima H, Asamoto S, Miki Y. Pathological mechanism and three-dimensional structure of cerebral dissecting aneurysms. J Neurosurg. 2001. 94: 712-7
9. Mizutani T, Kojima H, Asamoto S. Healing process for cerebral dissecting aneurysms presenting with subarachnoid hemorrhage. Neurosurgery. 2004. 54: 342-7
10. Mizutani T. Natural course of intracranial arterial dissections. J Neurosurg. 2011. 114: 1037-44
11. Murai Y, Matano F, Yokobori S, Onda H, Yokota H, Morita A. Treatment strategies of subarachnoid hemorrhage from bilateral vertebral artery dissection: A Case report and literature review focusing on the availability of stent placement. World Neurosurg. 2017. 106: 1050.e11-20
12. Nagahata M, Abe Y, Ono S, Hosoya T, Uno S. Surface appearance of the vertebrobasilar artery revealed on basiparallel anatomic scanning (BPAS)-MR imaging: Its role for brain MR examination. AJNR Am J Neuroradiol. 2005. 26: 2508-13
13. Naggara O, Louillet F, Touzé E, Roy D, Leclerc X, Mas JL. Added value of high-resolution MR imaging in the diagnosis of vertebral artery dissection. AJNR Am J Neuroradiol. 2010. 31: 1707-12
14. Naito I, Iwai T, Sasaki T. Management of intracranial vertebral artery dissections initially presenting without subarachnoid hemorrhage. Neurosurgery. 2002. 51: 930-7
15. Otawara Y, Ogasawara K, Ogawa A, Kogure T. Dissecting aneurysms of the bilateral vertebral arteries with subarachnoid hemorrhage: Report of three cases. Neurosurgery. 2002. 50: 1372-4
16. Ro A, Kageyama N, Abe N, Takatsu A, Fukunaga T. Intracranial vertebral artery dissection resulting in fatal subarachnoid hemorrhage: Clinical and histopathological investigations from a medicolegal perspective. J Neurosurg. 2009. 110: 948-54
17. Shin YS, Kim BM, Kim SH, Suh SH, Ryu CW, Koh JS. Endovascular treatment of bilateral intracranial vertebral artery dissecting aneurysms presenting with subarachnoid hemorrhage. Neurosurgery. 2012. 70: 75-81
18. Tsukahara T, Minematsu K. Overview of spontaneous cervicocephalic arterial dissection in japan. Acta Neurochir Suppl. 2010. 107: 35-40
19. Zhao WY, Zhao KJ, Huang QH, Xu Y, Hong B, Liu JM. Single-stage endovascular treatment of subarachnoid hemorrhage related to bilateral vertebral artery dissecting aneurysms. Interv Neuroradiol. 2016. 22: 138-42