- Department of Neurosurgery, Tokyo Medical University, Shinjukuku, Tokyo, Japan
Correspondence Address:
Jiro Akimoto
Department of Neurosurgery, Tokyo Medical University, Shinjukuku, Tokyo, Japan
DOI:10.4103/sni.sni_134_17
Copyright: © 2017 Surgical Neurology International This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Jiro Akimoto, Norio Ichimasu, Rei Haraoka, Shinjiro Fukami, Michihiro Kohno. A case of unruptured aneurysm of the internal carotid artery presenting as olfactory hallucinations. 22-Aug-2017;8:197
How to cite this URL: Jiro Akimoto, Norio Ichimasu, Rei Haraoka, Shinjiro Fukami, Michihiro Kohno. A case of unruptured aneurysm of the internal carotid artery presenting as olfactory hallucinations. 22-Aug-2017;8:197. Available from: http://surgicalneurologyint.com/surgicalint-articles/a-case-of-unruptured-aneurysm-of-the-internal-carotid-artery-presenting-as-olfactory-hallucinations/
Abstract
Background:Olfactory hallucination, a symptom of medial temporal lobe epilepsy, is rarely associated with unruptured intracranial aneurysms.
Case Description:We encountered this situation in a 70-year-old woman with an unruptured aneurysm at the bifurcation of the internal carotid and posterior communicating artery. We were able to achieve epileptic control by craniotomy clipping and medial temporal lesionectomy.
Conclusion:According to our knowledge, previous reports are limited to cases of large middle cerebral artery aneurysms compressing the lateral orbitofrontal cortex, and this is apparently the first report of a case where olfactory hallucinations occurred from direct stimulation of the entorhinal cortex by an internal carotid and posterior communicating artery bifurcation aneurysm. We examined the pathophysiology underlying the development of olfactory hallucinations. We found craniotomy clipping and focal resection to be useful from the standpoint of seizure control. Whether seizure control can also be obtained with intracranial aneurysm coiling should be investigated in the future.
Keywords: Neck clipping, olfactory hallucination, resection of epileptogenic foci, surgery, unruptured aneurysm
INTRODUCTION
Having olfactory hallucinations is a symptom of medial temporal lobe epilepsy syndrome. It is believed that they occur from stimulation of the region where the lateral olfactory stria passes through the amygdala to the entorhinal cortex in the anterior parahippocampal gyrus.
In this article, we report our experience with a patient, presenting with olfactory hallucinations, who underwent aneurysmal clipping and resection of epileptic foci for an unruptured aneurysm of the internal carotid artery.
CASE PRESENTATION
The patient was a 70-year-old woman. She had an elder sister with oculomotor nerve palsy who had undergone clipping for an internal carotid artery aneurysm. The patient was also concerned about an aneurysm but had never experienced a severe headache. She visited a local clinic because she suddenly developed the symptom of smelling burning rubber, which occurred a few times per week for the previous month. She underwent magnetic resonance imaging (MRI) of the head and was found to have a right internal carotid artery aneurysm. She was then referred and admitted to our hospital. She had clear consciousness, without any particular neurological deficits or any abnormalities in blood chemistry. Even after hospitalization, she had sudden episodes of phantosmia, which, however, were not associated with loss of consciousness. No obvious spikes were observed on scalp electroencephalogram (EEG). On magnetic resonance angiography (MRA) of the head, we noted a dumbbell-shaped aneurysm, 10 mm in diameter, appeared to have extended from the right internal carotid artery to pierce the medial temporal lobe cortex [
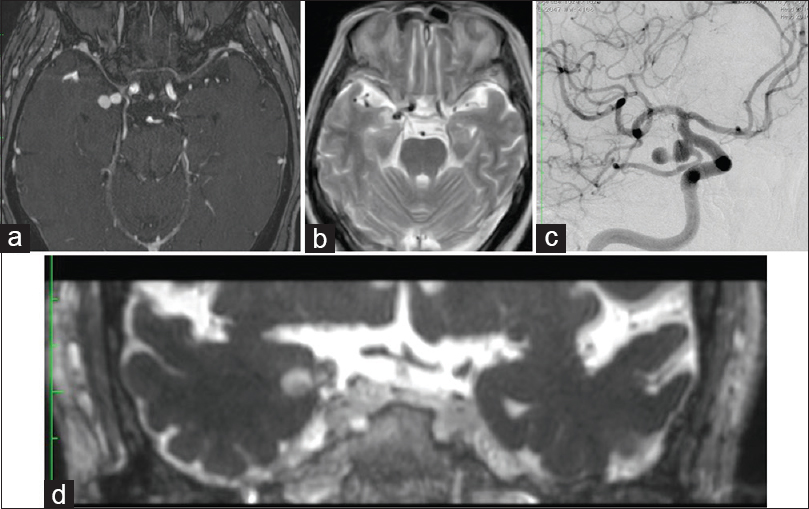
Figure 1
(a) An original image of gadolinium-enhanced MR angiography. A dumbbell-shaped aneurysm is intruding into the entorhinal sulcus from the right internal carotid artery. (b) A T2-weighted horizontal MRI image. A high signal intensity area suggesting edema around the aneurysm is shown in the medial temporal lobe. (c)The right internal carotid artery angiography shows an aneurysm with a narrow neck at the posterior communicating artery bifurcation. (d) A coronal section image of gadolinium-enhanced T2-weighted CISS MRI. The tip of the aneurysm reaches the vicinity of the amygdala nuclei
Surgery
A right pterional craniotomy was performed using a trans-sylvian approach to the right internal carotid artery. After confirmation of the presence of the aneurysm, 16 electrodes were placed over the lateral temporal lobe cortex to record temporal cortical EEG. As a result, clear spike discharges were detected from some electrodes [
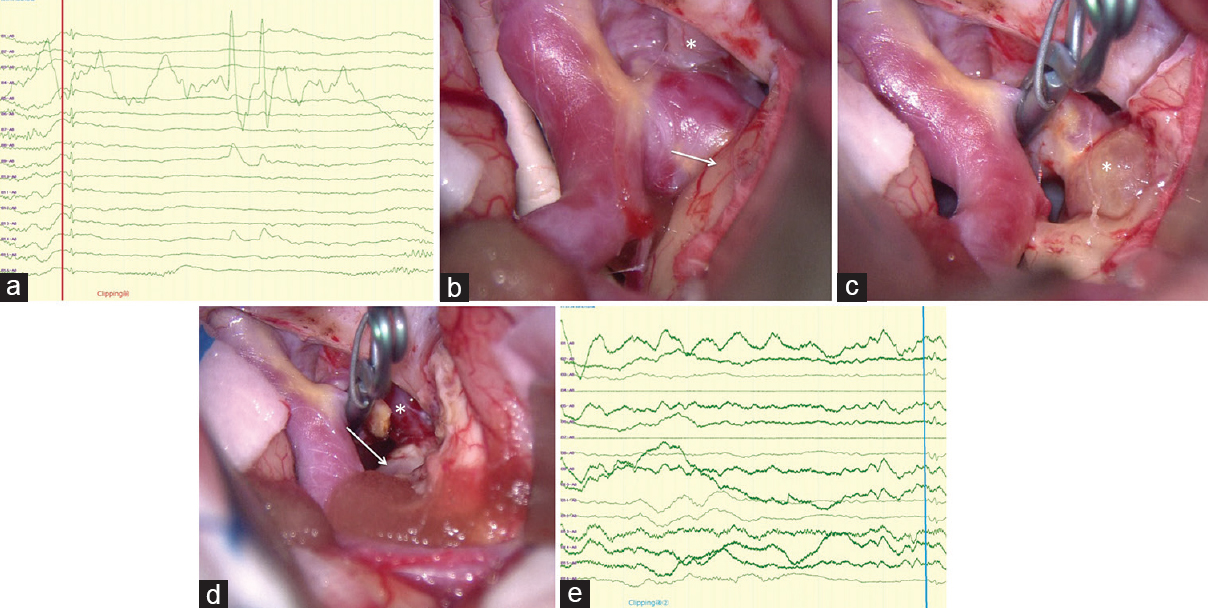
Figure 2
(a) Clear spikes were evoked in several electrodes of intraoperative cortical EEG. (b) The exposed aneurysm intruded into the right medial temporal lobe cortex, where brownish discoloration (arrow) occurred (*right oculomotor nerve). (c) After detachment of the posterior communicating artery and its perforators, neck clipping was performed, followed by corticotomy of the brownish discolored area of the medial temporal lobe cortex. With these procedures, the thrombosed distal dome (*) was exposed. (d) After resection of the aneurysmal dome, the right medial temporal lobe cortex was removed until normally colored white matter was exposed. (e) No obvious spikes were detected on the intraoperative cortical EEG after removal of the medial temporal lobe cortex
Pathology
The resected aneurysm wall was a pseudoaneurysm without an arterial wall structure; the internal elastic lamina was composed of thin fibrous tissue with a mild inflammatory response [
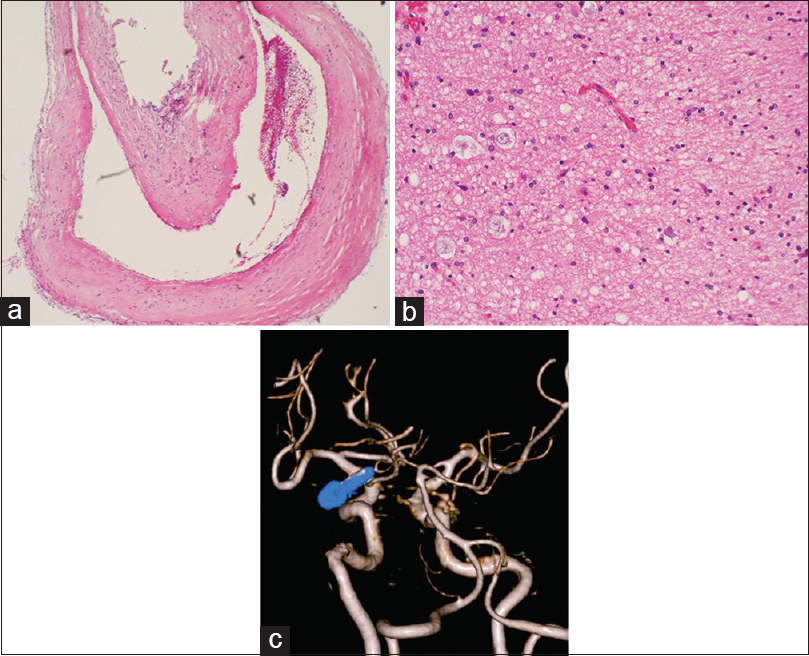
Figure 3
(a) The resected aneurysm wall was a pseudoaneurysm without an arterial wall structure. (b) The medial temporal lobe resected at the same time showed degenerated neurons and edematous tissue accompanied by gliosis. There was no clear hemosiderin deposition. (c) Postoperative 3D-CT angiography confirmed the complete disappearance of the aneurysm and presented a favorable image of the posterior communicating artery
Postoperative course
The patient had an uneventful clinical course, and the olfactory hallucinations resolved. Postoperative 3D-CT angiography confirmed the disappearance of the aneurysm and the patency of the P-com [
DISCUSSION
A study analyzing 1423 patients with partial epilepsy reported that olfactory hallucination was a rare symptom, occurring in only 13 patients (0.9%).[
Unruptured intracranial aneurysms rarely lead to partial seizures. According to a study conducted by Solomon et al. in 202 patients with unruptured intracranial aneurysms, 5% of the patients had seizures, and all of them had large aneurysms of ≥1 cm in diameter.[
Regarding the mechanism underlying the development of partial seizures due to unruptured intracranial aneurysms, potential causes are considered to be direct compression stimulation of the cortex by an aneurysm, minor hemorrhage from an aneurysm, or infarction caused by emboli from an aneurysm.[
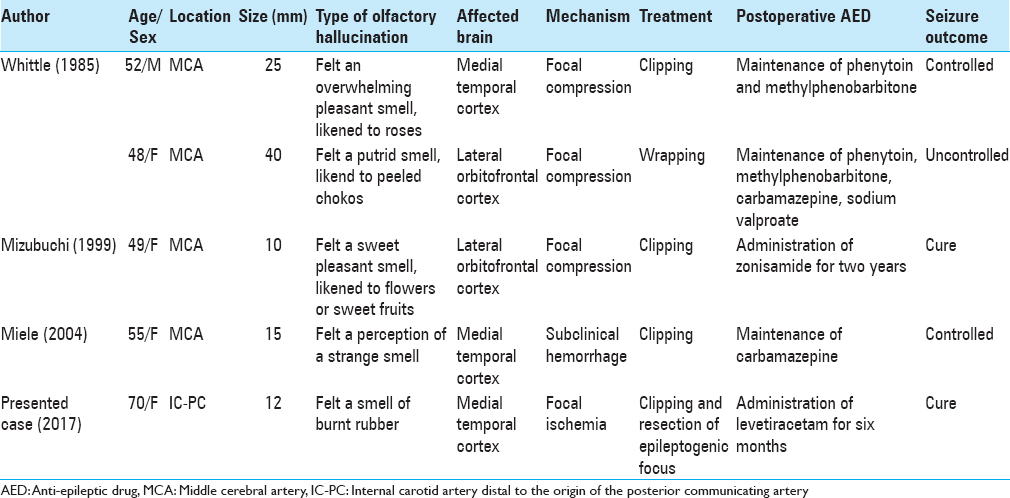
Majority of patients with unruptured intracranial aneurysms presenting with partial seizures progress to general convulsions. The occurrence of seizures in these patients is considered a warning sign of impending rupture of the aneurysm. Therefore, aneurysmal clipping is performed in most patients. However, the clipping alone, which can prevent the rupture, is considered inadequate from the standpoint of seizure control. Therefore, in the present case, we performed cortical EEG monitoring, resected the aneurysm following clipping, and then confirmed the disappearance of spikes on the cortical EEG after the macroscopically discolored area was completely removed. Consequently, the patient achieved a seizure-free state at the sixth postoperative month. In the literature, a study by Whittle et al.[
To accurately determine whether resection of epileptic foci is required for control of seizures in such cases would require a randomized controlled trial. Considering the rarity of these lesions, it is unlikely such a trial will ever be conducted, and therefore, decisions regarding the management of these patients will need to be made on an individual basis.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Acharya V, Acharya J, Luders H. Olfactory epileptic auras. Neurology. 1998. 51: 56-61
2. Chen C, Shih YH, Yen DJ, Lirng JF, Guo YC, Yu HY. Olfactory auras in patients with temporal lobe epilepsy. Epilepsia. 2003. 44: 257-60
3. Currie S, Heathfield KWG, Henson RA, Scott DF. Clinical course and prognosis of temporal lobe epilepsy survey of 666 patients. Brain. 1971. 94: 173-90
4. Kuba R, Krupa P, Okacova L, Rektor I. Unruptured intracranial aneurysm as a cause of focal epilepsy: An excellent postoperative outcome after intra-arterial treatment. Epileptic Disord. 2004. 6: 41-4
5. Miele VJ, Bendok BR, Batjer HH. Unruptured aneurysm of the middle cerebral artery presenting with psychomotor seizures: Case study and review of literature. Epilepsy Behav. 2004. 5: 420-8
6. Mizobuchi M, Ito N, Tanaka C, Sako K, Sumi Y, Sasaki T. Unidirectional olfactory hallucination associated with ipsilateral unruptured intracranial aneurysm. Epilepsia. 1999. 40: 516-9
7. Solomon RA, Fink ME, Pile-Spellman J. Surgical management of unruptured intracranial aneurysms. J Neurosurg. 1994. 80: 440-6
8. Whittle IR, Allsop JL, Halmagyi GM. Focal seizures: An unusual presentation of giant intracranial aneurysms: A report of four cases with comments on the natural history and treatment. Surg Neurol. 1985. 24: 533-40