- Department of Neurosurgery, Antônio Pedro Hospital Federal Fluminense University, Rio de Janeiro, Brazil
- Hot Springs Neurosurgical Clinic P.A, Hot Springs, Arkansas, USA
Correspondence Address:
Felipe de Oliveira
Hot Springs Neurosurgical Clinic P.A, Hot Springs, Arkansas, USA
DOI:10.4103/sni.sni_341_17
Copyright: © 2018 Surgical Neurology International This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Felipe de Oliveira, José Alberto Landeiro, Igor de Castro. Adult hemispheric cerebellar medulloblastoma. 14-Feb-2018;9:34
How to cite this URL: Felipe de Oliveira, José Alberto Landeiro, Igor de Castro. Adult hemispheric cerebellar medulloblastoma. 14-Feb-2018;9:34. Available from: http://surgicalneurologyint.com/surgicalint-articles/adult-hemispheric-cerebellar-medulloblastoma/
Abstract
Background:Medulloblastoma is an embryonal neoplasm and accounts for 1% of all adult intracranial tumors. It is associated with many familiar cancer syndromes, but there is no known cause for medulloblastoma. Many studies have documented differences between childhood and adult medulloblastomas in terms of location, proliferation, and apoptotic indices. There are four histological groups – classic and the variant forms (desmoplastic/nodular, anaplasic, and large cell). There are four major subgroups according to molecular configuration: wingless (WNT), sonic hedgehog (SHH), group 3, and group 4 with differences between them according to prognostic outcomes.
Case Description:We present the case of a 19-year-old female who complained of headache and vomiting. On neurological exam, she was awake, conscious, and had mild truncal ataxia, dysmetria, and intentional tremor. Brain magnetic resonance imaging (MRI) showed an intra-axial left hemisphere cerebellar lesion causing midline shift tonsilar herniation. She was submitted for posterior fossa craniotomy and microsurgical resection of cerebellar tumor and then to 18 Gy adjuvant radiotherapy to the tumor bed and 23 Gy to the neuroaxis.
Conclusion:This article briefly discusses the newest points in classification, diagnosis, and treatment of medulloblastoma. This case illustrates the diagnostic workup and treatment of a rare tumor in adults showing the importance of molecular and histological studies for the treatment and counseling of the patient. Medulloblastoma has different prognosis depending on the histological and molecular feature. Accessing these different features is essential to better plan the treatment as well as inform the patient regarding the disease and its prognosis.
Keywords: Brain tumor, medulloblastoma, molucular profile
INTRODUCTION
Medulloblastoma (MB) is an embryonal tumor. Most cases in this age group (adults) occur between the third and the fourth decade with a higher incidence in males. MB is associated with many familiar cancer syndromes, but there is no known cause for MB. Prenatal exposure to dietary N-nitroso compounds increases the risk. John Cunningham polyomavirus (JC Virus) T-antigen was identified in MB cells, suggesting a role of this viral infection in tumor physiopathology.
In the pediatric group, MB is the most common malignant brain tumor, but accounts for less than 1% of all adult intracranial tumors,[
CASE REPORT
We present the case of a 19-year-old female who complained of headache and vomiting. On neurological examination, she was awake, conscious, and had mild truncal ataxia, dysmetria, and intentional tremor.
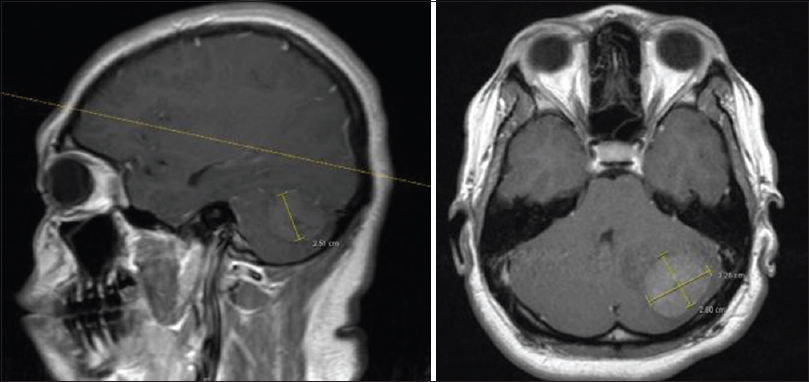
Brain magnetic resonance imaging (MRI) showed an intra-axial left hemisphere cerebellar lesion. One small area was visible within the mass. Severe vasogenic edema was noticed at the cerebellum causing mass effect, midline shift, and mild tonsilar herniation [
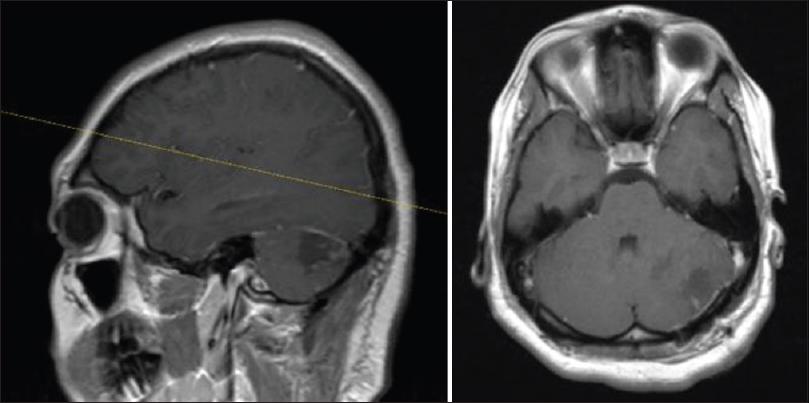
The patient underwent a posterior fossa craniotomy and microsurgical resection of cerebellar tumor [
A linear incision was made over the left side between the mastoid and inion. Skin and muscle were dissected all the way to the bone. A burr hole was perforated at the transverse sinus and another one lower down. The dura mater was easily dissected. Using craniotome, the two burr holes were connected and the bone flap was elevated. Dura was opened and elevated cranially, exposing the cerebellum. Using a superior approach to the cerebellum, the lesion was observed and resection was initiated with cavitron ultrasonic surgical aspirator (CUSA). The tumor was completely removed in a piece-meal fashion. Hemostasis was made using bipolar coagulation and injection of thrombin on the tumor bed. Dura mater was closed and a 2 × 2 piece of DuraGen® was placed over the dura. The bone flap was reapproximated and the fascia, muscle, and skin were closed. The patient was extubated and transferred to the intensive care unit (ICU) in a stable condition.
One month after the surgery, the patient was subjected to 18 Gy adjuvant radiotherapy to the tumor bed and 23 Gy to the neuroaxis. No chemotherapy was administered.
Pathology and molecular biology
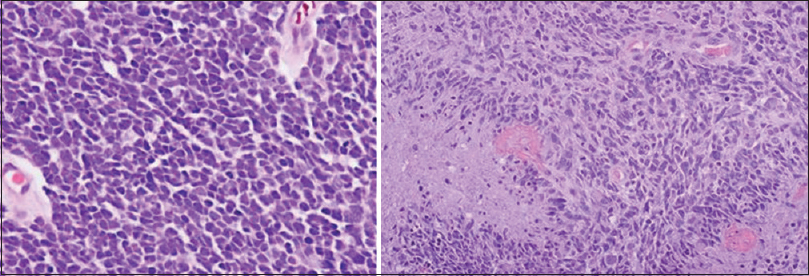
Histopathologic examination showed highly cellular neoplasm composed of large cells with hyperchromatic nuclei, scant cytoplasm, nuclear molding, and moderate pleomorphism, consistent with undifferentiated medulloblastoma [
On immunohistochemistry, the tumor was SHH subtype. No MYCN amplification or TP53 mutation were noted during molecular examinations.
DISCUSSION
Tumor biology
MB is a friable mass that arises most frequently in the cerebellar vermis, usually from the inferior medullary velum, but its presence in cerebellar hemispheres is not rare, especially in adults. Aqueduct of Sylvius and cisterna magna may be involved as the tumor grows. MB has a high propensity to metastasize commonly to the subarachnoid space along the brain and spinal cord. Metastatic spread has higher incidence in children than adults.[
There are four histological groups – classic and the variant forms (desmoplastic/nodular, anaplasic, and large cell).
Among MB variants, the most common is desmoplastic, especially in adults. It carries better prognostic than the classic form of MB. Large cell and anaplasic variants have a worse prognosis compared with the classic form.
Classic MB histological exam reveals small, round, basophilic cells with hyperchromatic nuclei, prominent nucleoli, and scant cytoplasm. Numerous mitotic figures are present. Homer–Wright rosettes may be occasionally found.
There are limited studies on the molecular subgrouping of adult MBs, recent expression profiling research have shown that MB is no longer considered a single disease entity, but has been shown to be a heterogeneous disease,[
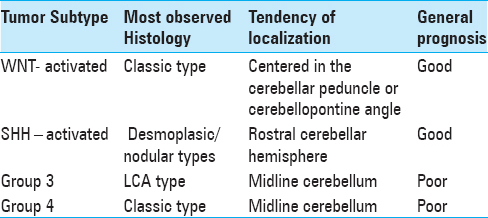
WNT subgroup of tumors displays predominantly classic histology, SHH tumors includes the desmoplastic/nodular, classic, large cell, and anaplasic subgroups. Group 3 and 4 tumors present as classic or highly aggressive large cell/anaplasic forms.[
Unlike in children where majority of tumors are located in the midline, in adults, approximately half of the adult tumors are located laterally in the cerebellar hemispheres, suggesting an alternative pathogenetic pathway with a different cell of origin.[
During embryonic growth, the cerebellum develops at the upper rhombic lip (URL) and the ventricular zone (VZ) surrounding the fourth ventricle. Upper rhombic lip originates all granule lineage cells of the external and internal granule layers (EGL and IGL, respectively).
During fetal development, on the cerebellar surface, granule neuron precursors (GNPs) multiply in the EGL, and then stop the cell cycle migrating inwards to the IGL, where they become post-mitotic granule neurons.
Recent researches have shown that desmoplastic MB tumors expressed markers associated with EGL-derived GNPs. Therefore, desmoplastic MBs is thought to originate from the GNPs in the EGL.
It is known that desmoplasic MB arises frequently from the cerebellar hemispheres, especially in adults, and that SHH subtype is common in the desmoplasic MB; hence, it is possible to have normal foci of GNPs in the EGL giving rise to desmoplasic SHH-MB.[
Recently, the 2016 WHO update of the WHO classification for CNS tumors removed the term primitive neuroectodermal tumor from the diagnostic lexicon. Therefore, tumors that once belonged to this group will now be referred to individually by name – medulloblastoma, atypical teratoid/rhabdoid tumors, and embryonal tumor with multilayered rosettes. According to the classification, MB is divided into its genetic subgroups.
MicroRNAs are small (approximately 22 nucleotide long) noncoding RNA molecules that play a key role in the post-transcriptional regulation of gene expression. These molecules bind to complementary sequences located on target mRNA and downregulate their expression by either causing degradation of the mRNA strand or suppressing translation [
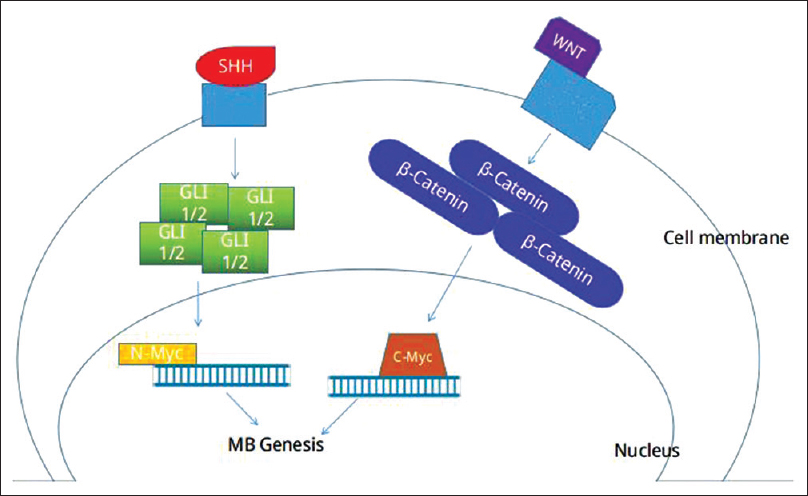
Figure 4
Scheme represents sinalization cascade for genesis of MB. Mutations in the genes that code for SHH signaling pathway receptors or downstream inhibitors may lead to hyperstimulation of the pathway, resulting in accumulation of oncogenic transcription factor GLI 1/2, activation of N-Myc and cellular deregulated proliferation. Mutations in genes that codify β-Catenin, results in accumulation of cytosolic β-catenin, activation of C-Myc and oncogenesis
The more aggressive subtypes of MB are associated with deregulation of a proto-oncogene family called MYC. The MYC proto-oncogene family includes three paralogs: c-MYC, MYCN, and MYCL. In MB, the most common paralog observed is MYCN, which encodes a transcription factor involved in controlling processes during embryonal development. Deregulated MYCN signaling supports the development of several different tumors, mainly with a childhood onset, including neuroblastoma, medulloblastoma, rhabdomyosarcoma, and Wilms’ tumor. MYCN amplification is the most consistent genetic aberration associated with poor prognosis and treatment failure.[
P53 is a tumor suppressor protein encoded by the TP53 gene. Many different types of cancer show a high incidence of TP53 mutations, leading to the expression of mutant p53 proteins. There is growing evidence that these mutant p53s have both lost wild-type p53 tumor suppressor activity and gained functions that help to contribute to malignant progression.[
Prognostic
Histopathological and molecular subtypes are useful prognostic factors for adult MB. These subtypes have a direct impact on the overall survival and progression-free survival rates with group 4 being associated with a high rate of high-risk disease, which may be the reason that this subgroup has the worst outcome among all the three subgroups.[
Different from early childhood cases where DNMBs have been linked to better survival than CMBs, no apparent difference seems to exist between DNMB and classic MB in adult. Tumors with anaplasic variants have a significantly worse outcome and are associated with molecular subtype group 4.[
On correlation of molecular subgroups with survival and progression-free survival, WNT subgroup has a better prognostic whereas SHH has an intermediate prognosis and group 4 has the worse prognostic.
Diagnostic workup
CT and MR scans are the gold standard tools for diagnosis. The spinal axis should be imaged at the time of diagnosis due to the high rates of neuroaxis dissemination.
In CT images, the tumor appears as a well-defined hyperdense mass in the vermis, although lesions in the cerebellar hemispheres can be found, especially in older patients. Any degree of enhancement may be found in postcontrast CT scans, but the most common pattern is a homogeneous intense enhancement. Hydrocephalus may be present in up to 90% of the cases.
MR is mandatory to evaluate tumor location, nearby infiltration, and subarachnoid dissemination. MB varies from hypointense to hyperintense on T2-weighted images and displays a heterogeneous pattern on T1-weighted MRI. Variable enhancement in gadolinium-enhanced scans is observed. MR is also important to evaluate the amount of residual tumor as it correlates to prognosis. To avoid the detection of nontumoral postoperative changes, MRI must be performed 48 h after the procedure.
CSF cytologic analysis helps defining subarachnoid dissemination and must be done together with spinal MRI in all cases of MB.
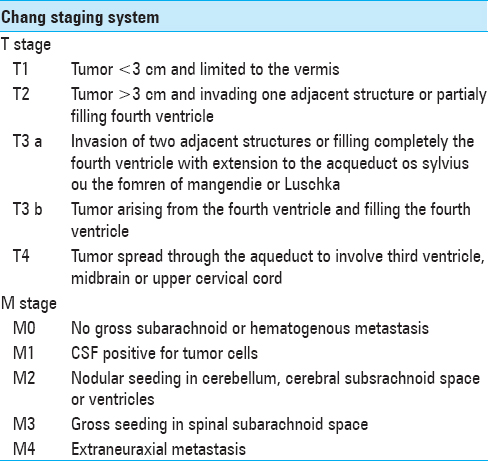
Staging
According to Chang's classification, patients are divided into average and high-risk groups, based in tumor size, invasion of nearby structures and subarachnoid dissemination [
Patients have high risk if metastases and/or residual mass (>1.5 cm2) are found after surgery and if there is unfavorable histology such as large cells/anaplasic variants. If none of these conditions are present, the risk is considered average.
Treatment
The ideal treatment for MB involves neurosurgical procedures from ventriculostomy to tumor resection. Ventriculostomy is often undertaken before resection to relieve high intracranial pressure caused by hydrocephalus. The most radical possible resection should be undertaken for a positive impact on prognosis.[
If hydrocephalus persists after tumor resection, a ventriculoperitoneal shunt or third ventriculostomy should be performed. Surgery alone is associated with a high incidence of recurrence.[
The impact of metastatic disease on 5-year progression-free survival (PFS) is well-established in literature. Chan et al. reported a 5-year progression free survival (PFS) rate of 47% and 59% in patients with and without metastatic dissemination, respectively.[
Radiotherapy is recommended for both average and high-risk patients and should start after 5 days of surgery. Delay and interruptions are associated with worse outcome.[
For high-risk patients, treatment involves craniospinal irradiation (36 Gy/20 fractions with posterior fossa boost of 19.8 Gy/11 fractions). Spinal metastatic disease requires higher spinal irradiation doses.
The efficacy of postoperative chemotherapy, with or without postoperative radiotherapy, has been well established in the pediatric population; however, there are insufficient data to define the use of chemotherapy in adults with average-risk disease. Therefore, this subset of patients is treated with adjuvant radiotherapy alone.
Radiotherapy may cause some side effects such as cognitive impairment, cerebellar mutism, endocrinological sequelae, and secondary tumors.
Cognitive impairment is observed in almost 70% of patients after treatment. Twenty-five percent of children with radio-induced cognitive impairment need special assistance in school.
Cerebellar mutism incidence is approximately 24%. Risk factors are tumor invasion of brainstem, medially localized tumor, and surgical lesion of toothed nucleus.
The risk of endocrine dysfunction enhances with the increase in radiation dose. The most affected hormone is growth hormone followed by thyroid hormone deficit.
Secondary tumors are rare with an estimated incidence of 4.2% in 10 years. The most common are meningiomas and high-grade gliomas.
There are differences in the indication of radiotherapy between adults and children less than 5 years old, due to the fact that neurotoxicity of radiation is higher in very young patients, so radiation side effects, especially cognitive sequela justified the attempt to develop new strategies to delay or avoid radiotherapy in this group of patients. The study HIT’87 confirmed the possibility to delay radiotherapy in less than 3 years old children. However, due to the high frequency of local relapse in classic or anaplasic/large cells MB, radiotherapy is mandatory for 18 months old or older children. Some authors state that radiotherapy must be given until 150 days after the diagnosis but the timing may vary between services.
In adults, radiotherapy is the consensus and must be initiated within 90 days after the surgery.[
There is no standard regimen or exact timing for chemotherapy in adult high-risk disease, although its use is consensus in this subset of patients. The most common drugs used are cisplatin or carboplatin and etoposide with or without cyclophosphamide.[
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Abacioglu U, Uzel O, Sengoz M, Turkan S, Ober A. Medulloblastoma in adults: Treatment results and prognostic factors. Int J Radiat Oncol Biol Phys. 2002. 54: 855-60
2. Albright AL, Wisoff JH, Zeltzer PM, Boyett JM, Rorke LB, Stanley P. Effects of medulloblastoma resections on outcome in children: A report from the Children's Cancer Group. Neurosurgery. 1996. 38: 265-71
3. Branavan M, Chitra V, Nicole M, Bradley W, Sandra E, Katrin S. Medulloblastoma stem cells: Modeling tumor heterogeneity. Cancer Lett. 2013. 338: 23-31
4. Brandes AA, Bartolotti M, Marucci G, Ghimenton C, Agati R, Fioravanti A. New perspectives in the treatment of adult medulloblastoma in the era of molecular oncology. Crit Rev Oncol Hematol. 2015. 94: 348-59
5. Carrie C, Lasset C, Alapetite C, Haie-Meder C, Hoffstetter S, Demaille MC. Multivariate analysis of prognostic factors in adult patients with medulloblastoma. Retrospective study of 156 patients. Cancer. 1994. 74: 2352-60
6. Chan AW, Tarbell NJ, Black PM, Louis DN, Frosch MP, Ancukiewicz M. Adult medulloblastoma: Prognostic factors and patterns of relapse. Neurosurgery. 2000. 47: 623-31
7. Ellison DW, Dalton J, Kocak M, Nicholson SL, Fraga C, Neale G. Medulloblastoma: Clinicopathological correlates of SHH, WNT, and non-SHH/WNT molecular subgroups. Acta Neuropathol. 2011. 121: 381-96
8. Ferrante L, Mastronardi L, Celli P, Acqui M, Cervoni L, Fortuna A. Medulloblastoma in adulthood. J Neurosurg Sci. 1991. 35: 23-30
9. Fiore R, Khudayberdiev S, Christensen M, Siegel G, Flavell SW, Kim TK. Mef2-mediated transcription of the miR379–410 cluster regulates activity-dependent dendritogenesis by fine-tuning Pumilio2 protein levels. EMBO J. 2009. 28: 697-710
10. Frost PJ, Laperriere NJ, Wong CS, Milosevic MF, Simpson WJ, Pintilie M. Medulloblastoma in adults. Int J Radiat Oncol Biol Phys. 1995. 32: 951-7
11. Fu Z, Hiroko O, Lei X, Felice G, Chunde L, Peng L. Molecular subgroups of adult medulloblastoma: A long-term single-institution study. Neuro-Oncology. 2016. 18: 982-90
12. Gibson P, Tong Y, Robinson G, Thompson MC, Currle DS, Eden C. Subtypes of medulloblastoma have distinct developmental origins. Nature. 2010. 468: 1095-9
13. Kavneet K, Aanchal K, Anupam K, Suvendu P, Supriya M, Vaishali S. Clinicopathological characteristics, molecular subgrouping, and expression of miR-379/miR-656 cluster (C14MC) in adult medulloblastomas. J Neurooncol. 2016. 130: 423-30
14. Korshunov A, Remke M, Werft W, Benner A, Ryzhova M, Witt H. Adult and pediatric medulloblastomas are genetically distinct and require different algorithms for molecular risk stratification. J Clin Oncol. 2010. 28: 3054-60
15. Muller PA, Vousden KH. Mutant p53 in Cancer: New Functions and Therapeutic Opportunities. Cancer Cell. 2014. 25: 304-17
16. Padovani L, Sunyach MP, Perol D, Mercier C, Alapetite C, Haie-Meder C. Common strategy for adult and pediatric medulloblastoma: A multicenter series of 253 adults. Int J Radiat Oncol Biol Phys. 2007. 68: 433-40
17. Rodriguez FJ, Eberhart C, O’Neill BP, Slezak J, Burger PC, Goldthwaite P. Histopathologic grading of adult medulloblastomas. Cancer. 2007. 109: 2557-65
18. Ruiz-Pérez MV, Henley AB, Arsenian-Henriksson M. The MYCN Protein in Health and Disease. Genes (Basel). 2017. p. 8-
19. Vidya G, Rong-H , Tara D, William B, Soumen K. Medulloblastoma development: Tumor biology informs treatment decisions. CNS Oncol. 2015. 4: 79-89
20. Vigneron C, Entz-Werlé N, Lutz P, Spiegel A, Jannier S, Helfre S. Evolution of the management of pediatric and adult medulloblastoma. Cancer Radiother. 2015. 19: 347-57
21. Winter J. MicroRNAs of the miR379–410 cluster: New players in embryonic neurogenesis and regulators of neuronal function. Neurogenesis (Austin). 2015. 2: e1004970-