- Section of Vascular Medicine, Cardiovascular Center, Lumberton, NC, USA
- School of Osteopathic Medicine, Campbell University, Lumberton, NC, USA
- Neurosurgeon, Neurological Center, Lumberton, NC, USA
Correspondence Address:
Saksith Smithason
Neurosurgeon, Neurological Center, Lumberton, NC, USA
DOI:10.4103/sni.sni_254_18
Copyright: © 2018 Surgical Neurology International This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.How to cite this article: Chompunut Asavaaree, Cara Doyle, Saksith Smithason. Artery of Percheron infarction results in severe bradycardia: A case report. 19-Nov-2018;9:230
How to cite this URL: Chompunut Asavaaree, Cara Doyle, Saksith Smithason. Artery of Percheron infarction results in severe bradycardia: A case report. 19-Nov-2018;9:230. Available from: http://surgicalneurologyint.com/surgicalint-articles/9071/
Abstract
Background:The thalamus is normally supplied by each posterior cerebral artery (PCA). The artery of percheron is a variant of this anatomy as it arises as a single trunk unilaterally from the PCA to supply the thalamus bilaterally. Occlusion of this artery is rare, and the diagnosis is usually missed without obtaining an MRI.
Case Description:We illustrate the case of a 68-year-old male who presented with coma, ocular gaze palsy, and severe bradycardia from bilateral thalamic nuclei and midbrain infarction, as described as an artery of Percheron infarction. The patient recovered neurologically under conservative treatment with a residual vertical diplopia from downward gaze palsy. He underwent cardiac pacer implantation for severe bradycardia at the end of his admission. The thalamic pathway associated with cardiac rhythm, especially the zona inserta, is discussed. Publications related to the artery of Percheron are reviewed.
Conclusion:Coma and ocular gaze palsy are the most common presentations following thalamic and midbrain ischemia from artery of Percheron infarction. To our knowledge, only a single case of artery of Percheron infarction with severe bradycardia has been reported in the past. Our case attested the role of thalamic nuclei controlling cardiac rhythm.
Keywords: Artery of Percheron, coma, severe bradycardia, thalamic infarct, Zonainserta
INTRODUCTION
The artery of Percheron arises as a single common trunk from one of the posterior cerebral arteries (PCA), and provides a bilateral arterial blood supply to the paramedian thalamic and rostral midbrain.[
Alterations of consciousness from a bilateral thalamic infarction is prevalent in the literature,[
CASE
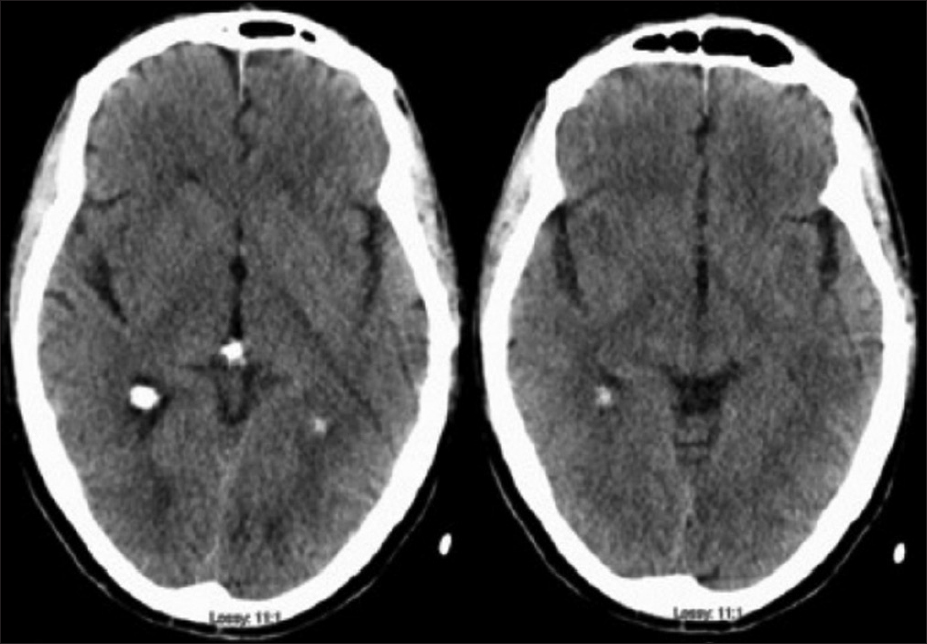
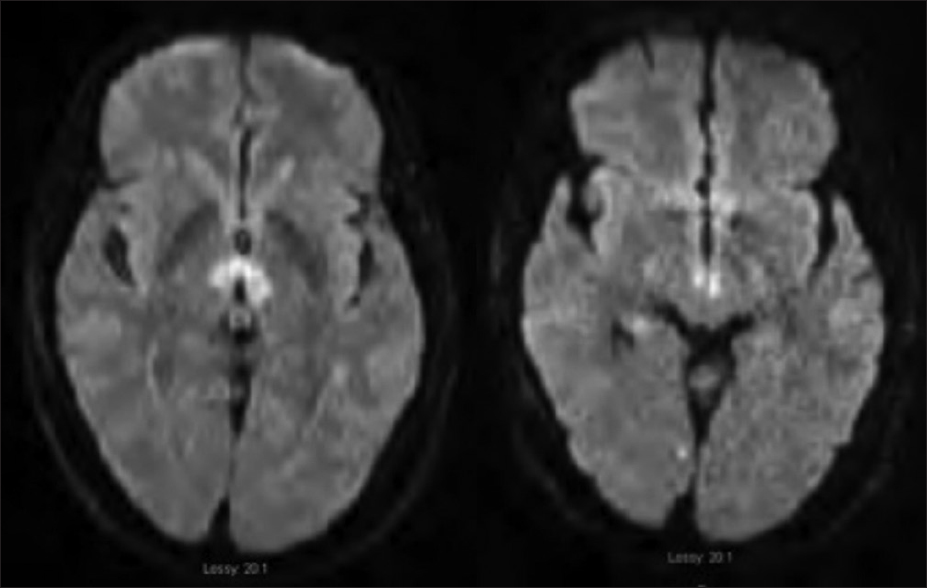
A 68-year-old, right-handed Native American male with a past medical history of hypertension and Gleason grade 3 prostate cancer presented to the emergency room with alterations of consciousness and severe bradycardia. His past surgical history included prostate cancer surgery and degenerative lumbar spine surgery. He is thin, athletic build with a BMI of 18.8. He is a non-smoker and has a baseline heart rate of 50 beats per minute. On the day of admission, he suddenly became unconscious while talking to his wife and fell from the chair. Less than five minutes later, he woke up and was confused. He was found incoherent upon arrival of the emergency medical team. His vitals were normal, and he was able to maintain his airway en route to the hospital. At the emergency room, his heart rate was 30–40 beats/min, blood pressure 120/78 mmHg, respiratory rate 16 breaths/min, and temperature 36.8° C. His oxygen saturation was above 92% on room air. Neurologically, his eyes were closed but opened in response to painful stimuli (E2, Glassgow Coma Score). He made noises and spoke incoherent words sporadically (V3, Glassgow Coma Score). He was not able to follow commands but was able to localize painful stimuli to his extremities equally bilaterally (M5, Glassgow Coma Score). His pupils were 3 mm in diameter and reactive to light. He was not alert enough to follow the extraocular muscle testing. His face was symmetric, and his reflexes were normal. Neither Babinski nor clonus sign were present. Given his initial Glasgow coma score of 10, he was intubated in the emergency room for airway protection. Code stroke was activated at the same time as a computer tomography (CT) head was obtained [
DISCUSSION
Although myocardial contraction is mainly controlled by the intrinsic sinoatrium and atrioventricular nodes, it also receives extrinsic neural input from anterior insula, posterior hypothalamus, rostral ventrolateral medulla, and the zona incerta.[
Consciousness is mediated by the RAS. Its nuclei are spread throughout the thalamus and brainstem, sending reciprocal fibers bilaterally to multiple areas of the cerebral cortex.[
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Arauz A, Patino-Rodriguez HM, Vargas-Gonzalez JC, Arguelles-Morales N, Silos H, Ruiz-Franco A. Clinical spectrum of artery of Percheron infarct: Clinical-radiological correlations. J Stroke Cerebrovasc Dis. 2014. 23: 1083-8
2. Aryan S, Thakar S, Hegde AS. Artery of Percheron infarction after endoscopic pituitary surgery. Acta Neurochir (Wien). 2016. 158: 1973-5
3. Bjornstad B, Goodman SH, Sirven JI, Dodick DW. Paroxysmal sleep as a presenting symptom of bilateral paramedian thalamic infarctions. Mayo Clin Proc. 2003. 78: 347-9
4. Edlow JA, Rabinstein A, Traub SJ, Wijdicks EFM. Diagnosis of reversible causes of coma. Lancet. 2014. 384: 2064-76
5. Jumean K, Arqoub AA, Al Hadidi MA, Hawatmeh A, Shaaban H. Bilateral thalamic stroke due to occlusion of the artery of Percheron in a patient with a patent foramen ovale. J Nat Sci Biol Med. 2016. 7: 109-12
6. Kocaeli H, Yilmazlar S, Kuytu T, Korfali E. The artery of Percheron revisited: Acadaveric anatomical study. Acta Neurochir (Wien). 2013. 155: 533-9
7. Lazzaro NA, Wright B, Castillo M, Fischbein NJ, Glastonbury CM, Hildenbrand PG. Artery of percheron infarction: Imaging patterns and clinical spectrum. AJNR Am J Neuroradiol. 2010. 31: 1283-9
8. Mayberg HS, Lozano AM, Voon V, McNeely HE, Seminowicz D, Hamani C. Deep brain stimulation for treatment-resistant depression. Neuron. 2005. 45: 651-60
9. Mazek H, Sherif K, Suarez J, Wischmeyer J. The artery of Percheron infarction after coronary angiography. Case Rep Cardiol 2016. 2016. p.
10. Mitrofanis J. Some certainty for the “zone of uncertainty”. Exploring the function of the zona incerta?. Neuroscience. 2005. 130: 1-15
11. Oppenheimer S. Cerebrogenic cardiac arrhythmias: Cortical lateralization and clinical significance. Clin Auton Res. 2006. 16: 6-11
12. Peruzzotti-Jametti L, Bacigaluppi M, Giacalone G, Strambo D, Comi G, Sessa M. Life-threatening bradycardia after bilateral paramedian thalamic and midbrain infarction. J Neurol. 2011. 258: 1895-7
13. Pitts-Tucker T, Small J. Artery of Percheron: An unusual stroke presentation. BMJ Case Rep. 2018. p.
14. Qian J, Wu C, Peng J, Liu H. Bilateral paramedian thalamic and midbrain infarction due to occlusion of the artery of percheron in an elderly male: Acase report. Neurol Sci. 2017. 38: 1123-6
15. Richardson L. Sick sinus syndrome. JAAPA. 2017. 30: 50-1
16. S Y-H. Acute cardiac sympathetic disruption in the pathogenesis of the takotsubo syndrome: Asystematic review of the literature to date. Cardiovasc Revasc Med. 2014. 15: 35-42
17. Spencer SE, SawyerWB , LoewyAD . L-glutamate stimulation of the zonaincerta in the rat decreases heart rate and blood pressure. Brain Res. 1988. 458: 72-81
18. Thayer JF, Lane RD. Claude Bernard and the heart-brain connection: Further elaboration of a model of neurovisceral integration. Neurosci Biobehav Rev. 2009. 33: 81-8
19. Thurtell MJ, Halmagyi GM. Complete ophthalmoplegia: An unusual sign of bilateral paramedian midbrain-thalamic infarction. Stroke. 2008. 39: 1355-7
20. Wong ML, Edlow JA. Artery of Percheron stroke. J Emerg Med. 2018. 55: 114-7
21. Yeo SS, Chang PH, Jang SH. The ascending reticular activating system from pontine reticular formation to the thalamus in the human brain. Front Hum Neurosci. 2013. 7: 416-