- Morton and Gloria Shulman Movement Disorders Clinic and the Edmond J. Safra Program in Parkinson's Disease, Toronto Western Hospital and Division of Neurology, University of Toronto, Toronto, Ontario, Canada
- Department of Neurology, Jackson Memorial Hospital and University of Miami Miller School of Medicine, Miami, Florida, USA
- Department of Neurosurgery, David Geffen School of Medicine at the University of California Los Angeles, Los Angeles, California, USA
- Krembil Research Institute, Toronto, Ontario, Canada
Correspondence Address:
Karlo J. Lizarraga
Morton and Gloria Shulman Movement Disorders Clinic and the Edmond J. Safra Program in Parkinson's Disease, Toronto Western Hospital and Division of Neurology, University of Toronto, Toronto, Ontario, Canada
Krembil Research Institute, Toronto, Ontario, Canada
DOI:10.4103/sni.sni_292_17
Copyright: © 2017 Surgical Neurology International This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Karlo J. Lizarraga, Corneliu C. Luca, Antonio De Salles, Alessandra Gorgulho, Anthony E. Lang, Alfonso Fasano. Asymmetric neuromodulation of motor circuits in Parkinson's disease: The role of subthalamic deep brain stimulation. 24-Oct-2017;8:261
How to cite this URL: Karlo J. Lizarraga, Corneliu C. Luca, Antonio De Salles, Alessandra Gorgulho, Anthony E. Lang, Alfonso Fasano. Asymmetric neuromodulation of motor circuits in Parkinson's disease: The role of subthalamic deep brain stimulation. 24-Oct-2017;8:261. Available from: http://surgicalneurologyint.com/surgicalint-articles/asymmetric-neuromodulation-of-motor-circuits-in-parkinsons-disease-the-role-of-subthalamic-deep-brain-stimulation/
Abstract
Whereas hemispheric dominance is well-established for appendicular motor control in humans, the evidence for dominance in axial motor control is still scarce. In Parkinson's disease (PD), unilateral (UL) onset of appendicular motor symptoms corresponds with asymmetric neurodegeneration predominantly affecting contralateral nigrostriatal circuits. Disease progression yields bilateral and axial motor symptoms but the initial appendicular asymmetry usually persists. Furthermore, there is evidence for hemispheric dominance for axial motor dysfunction in some of these patients. Dopaminergic medications improve appendicular symptoms but can also produce motor complications over time. Once these complications develop, bilateral (BL) deep brain stimulation (DBS) of the subthalamic nuclei (STN) can significantly improve appendicular symptoms while reducing medication doses and motor complications. Conversely, axial motor symptoms remain a significant source of disability, morbidity, and mortality for patients with PD. These axial symptoms do not necessarily improve with dopaminergic therapy, might not respond, and could even worsen after BL-DBS. In contrast to medications, DBS provides the opportunity to modify stimulation parameters for each cerebral hemisphere. Identical, BL-DBS of motor circuits with hemispheric dominance in PD might produce overstimulation on one side and/or understimulation on the other side, which could contribute to motor dysfunction. Several studies based on asymmetry of appendicular motor symptoms already support an initial UL rather than BL approach to DBS in some patients. The response of axial motor symptoms to UL versus BL-DBS remains unclear. Nonetheless, UL-DBS, staged BL-DBS, or asymmetric programming of BL-DBS could also be considered in patients with PD.
Keywords: Asymmetric neuromodulation, hemispheric dominance, Parkinson's disease, postural instability and gait dysfunction, subthalamic nucleus deep brain stimulation
INTRODUCTION
Parkinson's disease (PD) is a chronic neurodegenerative disorder associated with loss of dopaminergic neurons in the nigrostriatal pathways. In the U.S., approximately 1 million people have PD and additional 50,000 are diagnosed each year. The prevalence of PD significantly increases with age, ranging from 41 per 100,000 people in the 40–49 years group to 19 per 1,000 people older than 80 years worldwide.[
Appendicular motor dysfunction in early PD is usually unilateral (UL) and includes tremor, rigidity, and bradykinesia. Disease progression yields bilateral (BL) and axial motor symptoms, but the initial UL predominance usually persists. Dopaminergic agents can improve appendicular and some axial symptoms; however, they can also produce significant complications over time (fluctuations, dyskinesia). Once these complications develop, BL deep brain stimulation (DBS) of the subthalamic nuclei (STN) is a safe and effective intervention capable of improving appendicular symptoms while reducing medication requirements and motor complications. Axial motor dysfunction in PD includes dysarthria, dysphagia, postural instability, and gait dysfunction (PIGD) including freezing of gait (FOG). These symptoms remain a significant source of morbidity and mortality in PD. They do not necessarily improve with medications, might not respond, and could even worsen with BL STN-DBS.[
LATERALIZATION OF NORMAL MOTOR CONTROL
Certain brain functions are predominantly controlled by one hemisphere (i.e., hemispheric dominance or lateralization). For instance, the right (R) hemisphere is dominant for spatial cognition, body schema, proprioceptive control, and action inhibition,[
The mechanism of lateralization for motor control is unknown. Even though a genetic basis could be possible, heritability studies have not been conclusive.[
The L-sided lateralization for motor control is more evident for distal as opposed to proximal tasks, independent of the performing hand. Although motor dominance can be assessed in terms of preference (hand chosen to move) and/or performance (hand most proficient at the movement), it is also evident during bimanual movements. In this case, the dominant hand usually performs distal, fine movements, whereas the nondominant hand serves proximal, postural purposes.[
Though dominance for more proximal and axial motor functions such as posture, balance, and locomotion is not yet well-established, it has been proposed that the R hemisphere controls limb position and posture whereas the L hemisphere controls limb trajectory.[
Hemispheric functional differences for motor control have also been observed during learning. The progressive development of “motor expertise” has been associated with a transition from externally to internally generated movement control, along with a shift from R to L hemispheric activation. This phenomenon might be produced by a progressive reduction in the monitoring of global, spatial, and external features, as well as an increased representation of the local, internal motor program with learning.[
Even though locomotion is considered symmetric, functional gait asymmetries have been observed in normal humans.[
Because the functions preferentially carried out by the R hemisphere (visuospatial orientation, action inhibition, posture) are indispensable for movement,[
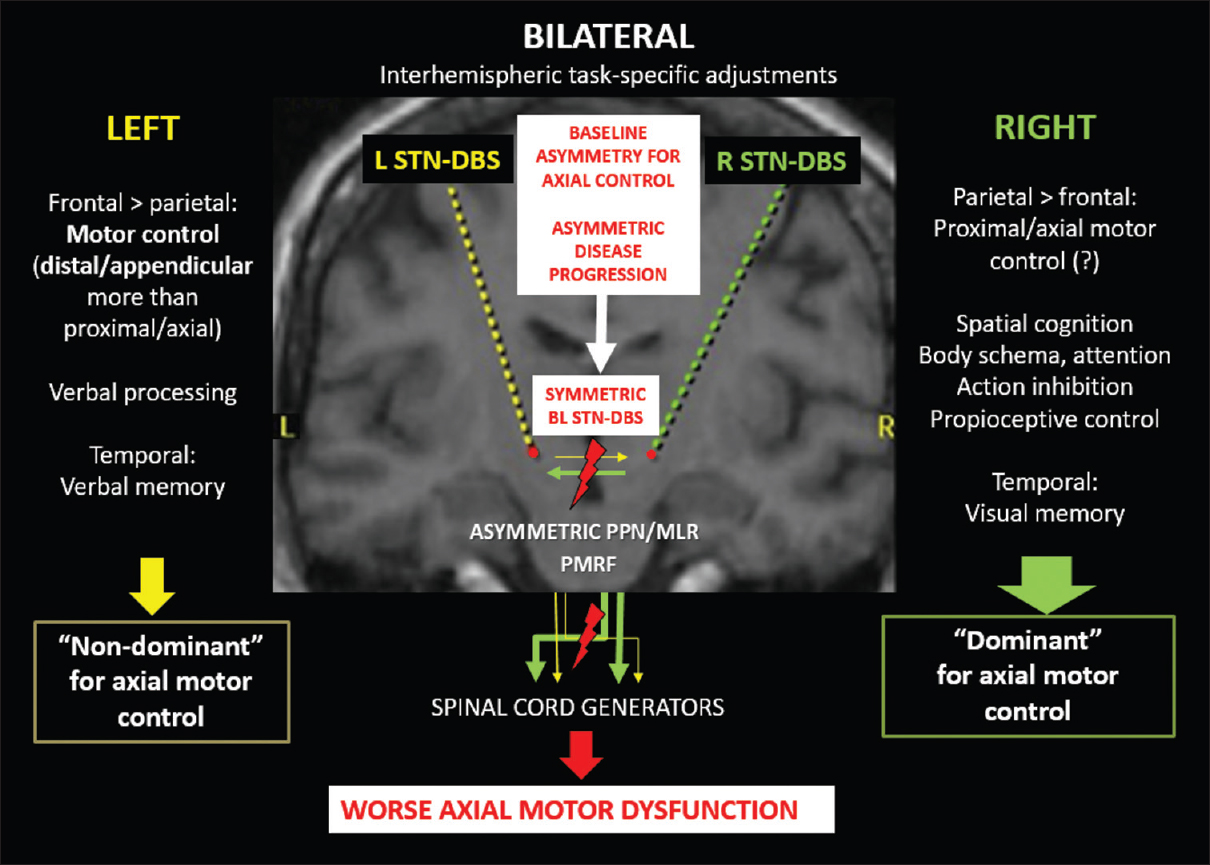
Figure 1
Graphic representation of the possible contributions of symmetric bilateral subthalamic stimulation to axial motor deterioration in the context of asymmetric circuits for axial motor control and Parkinson's disease progression. (L: left, R: right, BL: bilateral, PPN/MLR: pedunculopontine nucleus/mesencephalic locomotor region, PMRF: pontomedullary reticular formation)
LATERALIZATION OF MOTOR CONTROL IN PARKINSON'S DISEASE
Disease processes such as PD can also produce lateralized dysfunction. In fact, dopaminergic denervation of the striatum in PD begins asymmetrically and gradually becomes BL with disease progression.[
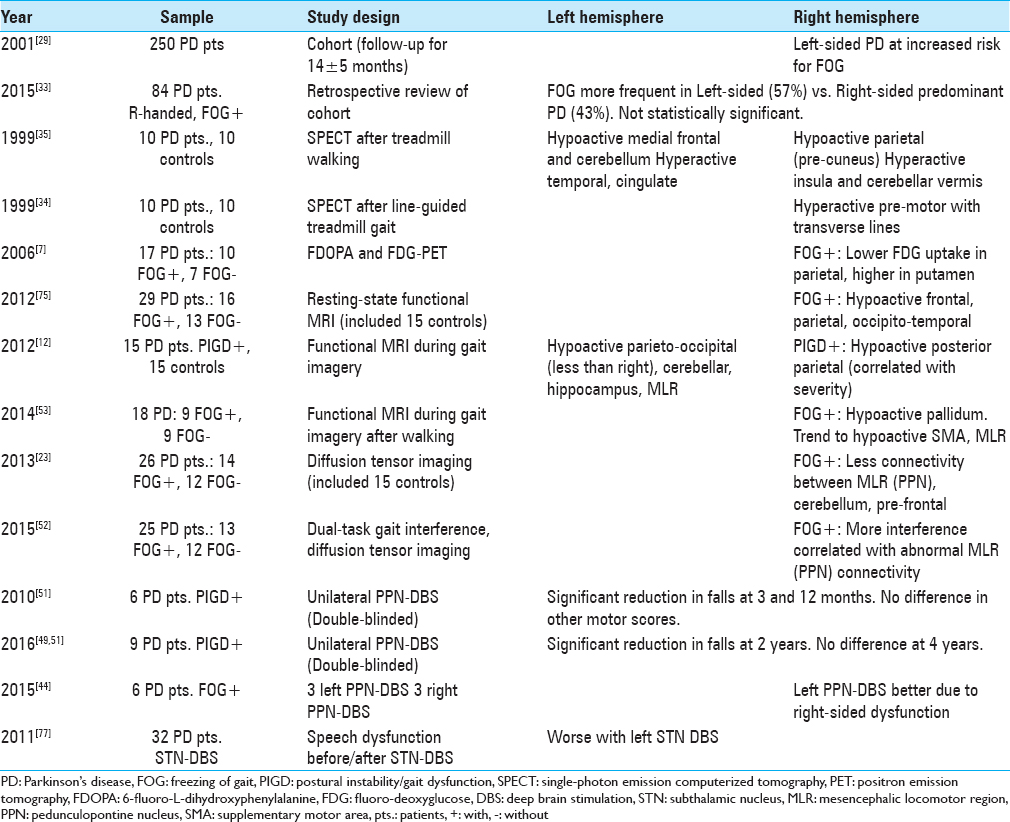
There is also evidence supporting lateralization of axial motor dysfunction in PD, which includes dysarthria, dysphagia, FOG, and PIGD.[
Patients with PD and FOG also appear to have abnormally reduced structural connectivity on diffusion tensor imaging (DTI) and functional MRI (fMRI) signals preferentially affecting R-sided motor circuits during gait imagery tasks.[
NEUROMODULATION OF LATERALIZED MOTOR CIRCUITS IN PARKINSON'S DISEASE
Dopaminergic agents can improve appendicular symptoms in PD; however, they can also produce motor fluctuations and/or dyskinesias over time. Once these complications develop, BL STN-DBS has emerged as a very effective and relatively safe treatment modality for these motor complications. It is especially effective in controlling appendicular symptoms in PD.[
The widespread BL as opposed to UL strategy for initial implantation of the STN is based on limited evidence. Identical DBS of potentially lateralized motor circuits might be unnecessary in all patients and could even contribute to the axial motor deterioration observed in some of them.[
Worsening of axial dysfunction after BL STN-DBS has been attributed to the variable combination of disease progression beyond dopaminergic systems and the unwanted spread of electrical fields beyond the STN.[
Interestingly, different patterns of local field potentials in motor networks have been associated with axial and appendicular symptoms. For instance, PIGD has been associated with decreased beta and increased gamma and alpha/theta bands. In contrast, bradykinesia has been associated with increased beta frequencies.[
In addition to PIGD, speech dysfunction can also be accelerated after BL STN-DBS in PD.[
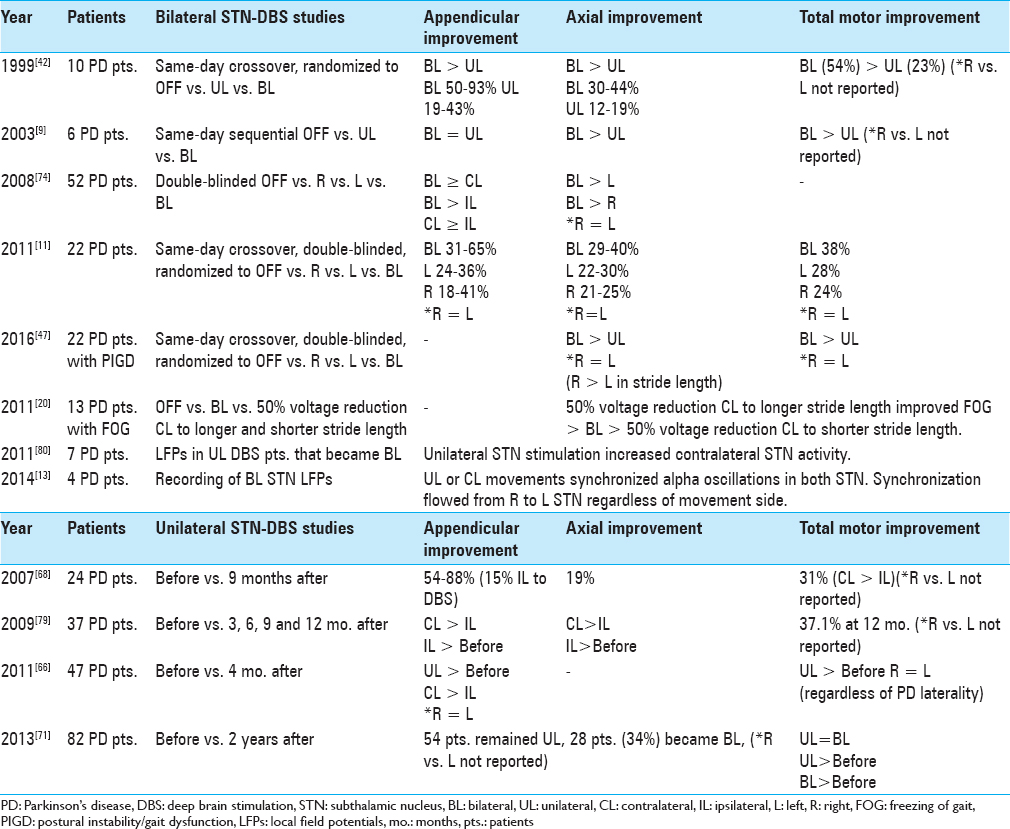
In contrast to medications, DBS provides the opportunity to modify some stimulation parameters separately for each cerebral hemisphere. In some patients, both appendicular and axial motor improvements achieved with UL and BL STN-DBS are similar.[
Based on the asymmetry of appendicular motor symptoms in PD, several studies support an initial UL as opposed to BL approach to STN-DBS in some patients [
Previous studies have suggested that UL and BL DBS can produce similar effects on axial symptoms in PD; however, BL stimulation appears to yield the maximal benefits.[
Given the established efficacy of BL STN-DBS and the significant contribution of axial dysfunction to morbidity and mortality in PD patients, it is paramount to identify patients whose axial dysfunction could worsen with BL as opposed to UL or individualized asymmetric programming of BL STN-DBS. A comprehensive presurgical assessment of DBS candidates that includes evaluation of motor lateralization could identify patients who would benefit from an initial UL as opposed to BL DBS paradigm based on both appendicular, axial, and possibly nonmotor symptoms. Although contralateral implantation might be required with disease progression, the initial UL approach could be maintained by asymmetric programming of DBS parameters for each hemisphere (e.g., voltage).[
CONCLUSIONS
Whereas the L hemisphere appears to be dominant for appendicular movements, there is growing evidence supporting R hemispheric dominance for axial motor control. Recently, theories involving complex interhemispheric relationships are attempting to unify the established models of hemispheric lateralization. In PD, BL STN-DBS is an established treatment modality that can significantly improve appendicular symptoms. Given the usually persistent asymmetry of appendicular symptoms in PD, some patients benefit from an asymmetric approach favoring DBS of the STN contralateral to the worse symptomatic side. Axial symptoms are still a significant contributor to disability, morbidity, and mortality in PD. These symptoms might not respond and can even worsen with BL STN-DBS. The comparative effects of UL or asymmetric programming of BL STN-DBS for axial symptoms have not yet been systematically evaluated, although data consistent with R-sided hemispheric dominance for axial control suggests that both UL and BL STN-DBS could produce similar effects. Thus, an asymmetric approach to STN-DBS implantation or programming could also be considered in PD patients with predominant axial dysfunction to avoid over- or understimulation of potentially asymmetric circuits. The availability of new DBS technology could facilitate the design of individualized asymmetric stimulation parameters that minimize axial impairment while maintaining appendicular symptom control.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Agostino R, Dinapoli L, Modugno N, Iezzi E, Gregori B, Esposito V. Ipsilateral sequential arm movements after unilateral subthalamic deep-brain stimulation in patients with Parkinson's disease. Mov Disord. 2008. 23: 1718-24
2. Alberts JL, Hass CJ, Vitek JL, Okun MS. Are two leads better than one: An emerging case for unilateral subthalamic deep brain stimulation in Parkinson's disease. Exp Neurol. 2008. 214: 1-5
3. Alberts JL, Okun MS, Vitek JL. The persistent effects of unilateral pallidal and subthalamic deep brain stimulation on force control in advanced Parkinson's patients. Parkinsonism Relat Disord. 2008. 14: 481-8
4. Alberts JL, Voelcker-Rehage C, Hallahan K, Vitek M, Bamzai R, Vitek JL. Bilateral subthalamic stimulation impairs cognitive-motor performance in Parkinson's disease patients. Brain. 2008. 131: 3348-60
5. Aron AR, Poldrack RA. Cortical and subcortical contributions to Stop signal response inhibition: Role of the subthalamic nucleus. J Neurosci. 2006. 26: 2424-33
6. Bagesteiro LB, Sainburg RL. Nondominant arm advantages in load compensation during rapid elbow joint movements. J Neurophysiol. 2003. 90: 1503-13
7. Bartels AL, de Jong BM, Giladi N, Schaafsma JD, Maguire RP, Veenma L. Striatal dopa and glucose metabolism in PD patients with freezing of gait. Mov Disord. 2006. 21: 1326-32
8. Bartels AL, Leenders KL. Brain imaging in patients with freezing of gait. Mov Disord. 2008. 23: S461-7
9. Bastian AJ, Kelly VE, Revilla FJ, Perlmutter JS, Mink JW. Different effects of unilateral versus bilateral subthalamic nucleus stimulation on walking and reaching in Parkinson's disease. Mov Disord. 2003. 18: 1000-7
10. Brooks DJ. Morphological and functional imaging studies on the diagnosis and progression of Parkinson's disease. J Neurol. 2000. 247: II11-8
11. Castrioto A, Meaney C, Hamani C, Mazzella F, Poon YY, Lozano AM. The dominant-STN phenomenon in bilateral STN DBS for Parkinson's disease. Neurobiol Dis. 2011. 41: 131-7
12. Cremers J, D’Ostilio K, Stamatakis J, Delvaux V, Garraux G. Brain activation pattern related to gait disturbances in Parkinson's disease. Mov Disord. 2012. 27: 1498-505
13. Darvas F, Hebb AO. Task specific inter-hemispheric coupling in human subthalamic nuclei. Front Hum Neurosci. 2014. 8: 701-
14. de la Fuente-Fernandez R, Kishore A, Calne DB, Ruth TJ, Stoessl AJ. Nigrostriatal dopamine system and motor lateralization. Behav Brain Res. 2000. 112: 63-8
15. Debaere F, Wenderoth N, Sunaert S, Van Hecke P, Swinnen SP. Changes in brain activation during the acquisition of a new bimanual coordination task. Neuropsychologia. 2004. 42: 855-67
16. Deuschl G, Schade-Brittinger C, Krack P, Volkmann J, Schafer H, Botzel K. A randomized trial of deep-brain stimulation for Parkinson's disease. N Engl J Med. 2006. 355: 896-908
17. di Biase L, Fasano A. Low-frequency deep brain stimulation for Parkinson's disease: Great expectation or false hope?. Mov Disord. 2016. 31: 962-7
18. Dorsey ER, Constantinescu R, Thompson JP, Biglan KM, Holloway RG, Kieburtz K. Projected number of people with Parkinson disease in the most populous nations, 2005 through 2030. Neurology. 2007. 68: 384-6
19. Fasano A, Aquino CC, Krauss JK, Honey CR, Bloem BR. Axial disability and deep brain stimulation in patients with Parkinson disease. Nat Rev Neurol. 2015. 11: 98-110
20. Fasano A, Herzog J, Seifert E, Stolze H, Falk D, Reese R. Modulation of gait coordination by subthalamic stimulation improves freezing of gait. Mov Disord. 2011. 26: 844-51
21. Ferraye MU, Debu B, Fraix V, Xie-Brustolin J, Chabardes S, Krack P. Effects of subthalamic nucleus stimulation and levodopa on freezing of gait in Parkinson disease. Neurology. 2008. 70: 1431-7
22. Fleury V, Pollak P, Gere J, Tommasi G, Romito L, Combescure C. Subthalamic stimulation may inhibit the beneficial effects of levodopa on akinesia and gait. Mov Disord. 2016. 31: 1389-97
23. Fling BW, Cohen RG, Mancini M, Nutt JG, Fair DA, Horak FB. Asymmetric pedunculopontine network connectivity in parkinsonian patients with freezing of gait. Brain. 2013. 136: 2405-18
24. Frank MJ, Samanta J, Moustafa AA, Sherman SJ. Hold your horses: Impulsivity, deep brain stimulation, and medication in parkinsonism. Science. 2007. 318: 1309-12
25. Franz EA, Eliassen JC, Ivry RB, Gazzaniga MS. Dissociation of spatial and temporal coupling in the bimanual movements of callosotomy patients. Psychol Sci. 1996. 7: 306-10
26. Galaburda AM, LeMay M, Kemper TL, Geschwind N. Right-left asymmetries in the brain. Science. 1978. 199: 852-6
27. Germano IM, Gracies JM, Weisz DJ, Tse W, Koller WC, Olanow CW. Unilateral stimulation of the subthalamic nucleus in Parkinson disease: A double-blind 12-month evaluation study. J Neurosurg. 2004. 101: 36-42
28. Geschwind N, Galaburda AM.editorsCerebral lateralization: Biological mechanisms, associations and pathology. Cambridge (MA): MIT Press; 1987. p.
29. Giladi N, McDermott MP, Fahn S, Przedborski S, Jankovic J, Stern M. Freezing of gait in PD: Prospective assessment in the DATATOP cohort. Neurology. 2001. 56: 1712-21
30. Glick SD, Ross DA, Hough LB. Lateral asymmetry of neurotransmitters in human brain. Brain Res. 1982. 234: 53-63
31. Goble DJ, Brown SH. The biological and behavioral basis of upper limb asymmetries in sensorimotor performance. Neurosci Biobehav Rev. 2008. 32: 598-610
32. Goldberg E, Podell K, Lovell M. Lateralization of frontal lobe functions and cognitive novelty. J Neuropsychiatry Clin Neurosci. 1994. 6: 371-8
33. Hall JM, Gilat M, Lewis SJ, Shine JM. Does dominant pedunculopontine nucleus exist? Probably not. Brain. 2015. 138: e346-
34. Hanakawa T, Fukuyama H, Katsumi Y, Honda M, Shibasaki H. Enhanced lateral premotor activity during paradoxical gait in Parkinson's disease. Ann Neurol. 1999. 45: 329-36
35. Hanakawa T, Katsumi Y, Fukuyama H, Honda M, Hayashi T, Kimura J. Mechanisms underlying gait disturbance in Parkinson's disease: A single photon emission computed tomography study. Brain. 1999. 122: 1271-82
36. Hershey T, Revilla FJ, Wernle A, Gibson PS, Dowling JL, Perlmutter JS. Stimulation of STN impairs aspects of cognitive control in PD. Neurology. 2004. 62: 1110-4
37. Hornykiewicz O. Dopamine (3-hydroxytyramine) and brain function. Pharmacol Rev. 1966. 18: 925-64
38. Ivry RB, Roberson LC.editorsThe Two Sides of Perception. Cambridge (MA): MIT Press; 1998. p.
39. Kempster PA, Gibb WR, Stern GM, Lees AJ. Asymmetry of substantia nigra neuronal loss in Parkinson's disease and its relevance to the mechanism of levodopa related motor fluctuations. J Neurol Neurosurg Psychiatry. 1989. 52: 72-6
40. Kim SG, Ashe J, Hendrich K, Ellerman JM, Merkle H, Ugurbil K. Functional magnetic resonance imaging of motor cortex: Hemispheric asymmetry and handedness. Science. 1993. 261: 615-7
41. Kooistra CA, Heilman KM. Motor dominance and lateral asymmetry of the globus pallidus. Neurology. 1988. 38: 388-90
42. Kumar R, Lozano AM, Sime E, Halket E, Lang AE. Comparative effects of unilateral and bilateral subthalamic nucleus deep brain stimulation. Neurology. 1999. 53: 561-6
43. Krack P, Batir A, Van Blercom N, Chabardes S, Fraix V, Ardouin C. Five-year follow-up of bilateral stimulation of the subthalamic nucleus in advanced Parkinson's disease. N Engl J Med. 2003. 349: 1925-34
44. Lam S, Moro E, Poon YY, Lozano AM, Fasano A. Does dominant pedunculopontine nucleus exist?. Brain. 2015. 138: e323-
45. Liang J, Hu X, Zhou X, Jiang X, Cao Y, Wang L. Five-year follow-up of 23 asymmetrical Parkinson's disease patients treated with unilateral subthalamic nucleus stimulation. Neural Regen Res. 2012. 7: 1428-35
46. Linazasoro G, Van Blercom N, Lasa A. Unilateral subthalamic deep brain stimulation in advanced Parkinson's disease. Mov Disord. 2003. 18: 713-6
47. Lizarraga KJ, Jagid JR, Luca CC. Comparative effects of unilateral and bilateral subthalamic nucleus deep brain stimulation on gait kinematics in Parkinson's disease: A randomized, blinded study. J Neurol. 2016. 263: 1652-6
48. Mestre TA, Sidiropoulos C, Hamani C, Poon YY, Lozano AM, Lang AE. Long-term double-blinded unilateral pedunculopontine area stimulation in Parkinson's disease. Mov Disord. 2016. 31: 1570-4
49. Moreau C, Defebvre L, Destee A, Bleuse S, Clement F, Blatt JL. STN-DBS frequency effects on freezing of gait in advanced Parkinson disease. Neurology. 2008. 71: 80-4
50. Moro E, Scerrati M, Romito LM, Roselli R, Tonali P, Albanese A. Chronic subthalamic nucleus stimulation reduced medication requirements in Parkinson's disease. Neurology. 1999. 53: 85-90
51. Moro E, Hamani C, Poon YY, Al Khairallah T, Dostrovsky JO, Hutchinson WD. Unilateral pedunculopontine stimulation improves falls in Parkinson's disease. Brain. 2010. 133: 215-24
52. Peterson DS, Fling BW, Mancini M, Cohen RG, Nutt JG, Horak FB. Dual-task interference and brain structural connectivity in people with Parkinson's disease who freeze. J Neurol Neurosurg Psychiatry. 2015. 86: 786-92
53. Peterson DS, Pickett KA, Duncan R, Perlmutter J, Earhart GM. Gait-related brain activity in people with Parkinson disease with freezing of gait. PLoS One. 2014. 9: e90634-
54. Petraglia FW, Farber SH, Han JL, Verla T, Gallis J, Lokhnygina Y. Comparison of Bilateral vs. Staged Unilateral Deep Brain Stimulation (DBS) in Parkinson's Disease in Patients Under 70 Years of Age. Neuromodulation. 2016. 19: 31-7
55. Pinto Y, Neville DA, Otten M, Corballis PM, Lamme VA, de Haan EH. Split brain: Divided perception but undivided consciousness. Brain. 2017. p.
56. Plotnik M, Giladi N, Balash Y, Peretz C, Hausdorff JM. Is freezing of gait in Parkinson's disease related to asymmetric motor function?. Ann Neurol. 2005. 57: 656-63
57. Pringsheim T, Jette N, Frolkis A, Steeves TD. The prevalence of Parkinson's disease: A systematic review and meta-analysis. Mov Disord. 2014. 29: 1583-90
58. Rossi A, Berger K, Chen H, Leslie D, Mailman RB, Huang X. Projection of the prevalence of Parkinson's disease in the coming decades: Revisited. Mov Disord. 2017. p.
59. Sadeghi H, Allard P, Prince F, Labelle H. Symmetry and limb dominance in able-bodied gait: A review. Gait Posture. 2000. 12: 34-45
60. Sadeghi H. Local or global asymmetry in gait of people without impairments. Gait Posture. 2003. 17: 197-204
61. Sainburg RL. Evidence for a dynamic-dominance hypothesis of handedness. Exp Brain Res. 2002. 142: 241-58
62. Sammartino F, Krishna V, King NK, Bruno V, Kalia S, Hodaie M. Sequence of electrode implantation and outcome of deep brain stimulation for Parkinson's disease. J Neurol Neurosurg Psychiatry. 2016. 87: 859-63
63. Schuepbach WM, Rau J, Knudsen K, Volkmann J, Krack P, Timmermann L. Neurostimulation for Parkinson's disease with early motor complications. N Engl J Med. 2013. 368: 610-22
64. Sergent J. The cerebral balance of power: Confrontation or cooperation?. J Exp Psychol Hum Percept Perform. 1982. 8: 253-72
65. Serrien DJ, Ivry RB, Swinnen SP. Dynamics of hemispheric specialization and integration in the context of motor control. Nat Rev Neurosci. 2006. 7: 160-6
66. Shemisa K, Hass CJ, Foote KD, Okun MS, Wu SS, Jacobson CE. Unilateral deep brain stimulation surgery in Parkinson's disease improves ipsilateral symptoms regardless of laterality. Parkinsonism Relat Disord. 2011. 17: 745-8
67. Singh A, Kammermeier S, Plate A, Mehrkens JH, Ilmberger J, Botzel K. Pattern of local field potential activity in the globus pallidus internum of dystonic patients during walking on a treadmill. Exp Neurol. 2011. 232: 162-7
68. Slowinski JL, Putzke JD, Uitti RJ, Lucas JA, Turk MF, Kall BA. Unilateral deep brain stimulation of the subthalamic nucleus for Parkinson disease. J Neurosurg. 2007. 106: 626-32
69. St. George RJ, Nutt JG, Burchiel KJ, Horak FB. A meta-regression of the long-term effects of deep brain stimulation on balance and gait in PD. Neurology. 2010. 75: 1292-9
70. Strafella A, Lozano AM, Ballanger B, Poon Y, Lang AE, Moro E. rCBF changes associated with PPN stimulation in a patient with Parkinson's disease: A PET study. Mov Disord. 2008. 23: 1051-4
71. Sung VW, Watts RL, Schrandt CJ, Guthrie S, Wang D, Amara AW. The relationship between clinical phenotype and early staged bilateral deep brain stimulation in Parkinson disease. J Neurosurg. 2013. 119: 1530-6
72. Swinnen SP, Jardin K, Meulenbroek R. Between-limb asynchronies during bimanual coordination: Effects of manual dominance and attentional cueing. Neuropsychologia. 1996. 34: 1203-13
73. Swinnen SP. Intermanual coordination: From behavioral principles to neural-network interactions. Nature Rev Neurosci. 2002. 3: 348-59
74. Tabbal SD, Ushe M, Mink JW, Revilla FJ, Wernle AR, Hong M. Unilateral subthalamic nucleus stimulation has a measurable ipsilateral effect on rigidity and bradykinesia in Parkinson disease. Exp Neurol. 2008. 211: 234-42
75. Tessitore A, Amboni M, Esposito F, Russo A, Picillo M, Marcuccio L. Resting-state brain connectivity in patients with Parkinson's disease and freezing of gait. Parkinsonism Relat Disord. 2012. 18: 781-7
76. Tommasi G, Lopiano L, Zibetti M, Cinquepalmi A, Fronda C, Bergamasco B. Freezing and hypokinesia of gait induced by stimulation of the subthalamic region. J Neurol Sci. 2007. 258: 99-103
77. Tripoliti E, Zrinzo L, Martinez-Torres I, Frost E, Pinto S, Foltynie T. Effects of subthalamic stimulation on speech of consecutive patients with Parkinson disease. Neurology. 2011. 76: 80-6
78. van Neunen BF, Esselink RA, Munneke M, Speelman JD, van Laar T, Bloem BR. Postoperative gait deterioration after bilateral subthalamic nucleus stimulation in Parkinson's disease. Mov Disord. 2008. 23: 2404-6
79. Walker HC, Watts RL, Guthrie S, Wang D, Guthrie BL. Bilateral effects of unilateral subthalamic deep brain stimulation on Parkinson's disease at 1 year. Neurosurgery. 2009. 65: 302-9
80. Walker HC, Watts RL, Schrandt CJ, Huang H, Guthrie SL, Guthrie BL. Activation of subthalamic neurons by contralateral subthalamic deep brain stimulation in Parkinson disease. J Neurophysiol. 2011. 105: 1112-21
81. Wang J, Yang QX, Sun X, Vesek J, Mosher Z, Vasavada M. MRI evaluation of asymmetry of nigrostriatal damage in the early stage of early-onset Parkinson's disease. Parkinsonism Relat Disord. 2015. 21: 590-6
82. Winstein CJ, Pohl PS. Effects of unilateral brain damage on the control of goal-directed hand movements. Exp Brain Res. 1995. 105: 163-74
83. Wolpert DM, Goodbody SJ, Husain M. Maintaining internal representations: The role of the human superior parietal lobe. Nat Neurosci. 1998. 1: 529-33
84. Zibetti M, Moro E, Krishna V, Sammartino F, Picillo M, Munhoz RP. Low-frequency Subthalamic Stimulation in Parkinson's Disease: Long-term Outcome and Predictors. Brain Stimul. 2016. 9: 774-9