- Department of Neurological Surgery, Juntendo University Urayasu Hospital, Urayasu, Chiba, Japan
Correspondence Address:
Satoshi Tsutsumi
Department of Neurological Surgery, Juntendo University Urayasu Hospital, Urayasu, Chiba, Japan
DOI:10.4103/sni.sni_37_18
Copyright: © 2018 Surgical Neurology International This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.How to cite this article: Satoshi Tsutsumi, Satoshi Adachi, Hisato Ishii, Yukimasa Yasumoto. Atypical epidural hemangiopericytoma presenting with visual disturbance. 05-Apr-2018;9:69
How to cite this URL: Satoshi Tsutsumi, Satoshi Adachi, Hisato Ishii, Yukimasa Yasumoto. Atypical epidural hemangiopericytoma presenting with visual disturbance. 05-Apr-2018;9:69. Available from: http://surgicalneurologyint.com/surgicalint-articles/atypical-epidural-hemangiopericytoma-presenting-with-visual-disturbance/
Abstract
Background:Hemangiopericytomas are a rare entity commonly presenting as subdural tumors.
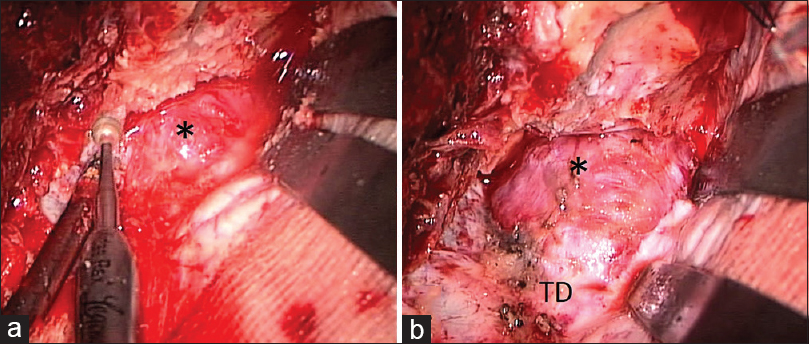
Case Description:A 57-year-old man presented with a progressive visual disturbance over a period of 3 weeks. Cranial computed tomography scans revealed an isodense mass at the tip of the left middle fossa, extending into the orbital apex, and accompanying bony erosions in the sphenoid ridge. On magnetic resonance imaging, the lesion appeared isointense both on T1- and T2-weighted sequences, and intensely enhanced on contrast examinations. A frontotemporal craniotomy revealed a dura-based, capsulized tumor located entirely in the epidural space. A gross total resection was achieved for the tumor and histologically verified as hemangiopericytoma.
Conclusion:Hemangiopericytoma should be assumed in a differential diagnosis when encountering epidural tumors, and total resection should be attempted when possible.
Keywords: Epidural, meningeal hemangiopericytoma, treatment, visual disturbance
INTRODUCTION
Hemangiopericytomas (HPs) are a rare entity that account for only 1% of all intracranial tumors.[
CASE PRESENTATION
A 57-year-old man presented with a progressive visual disturbance over a period of 3 weeks. His medical history was unremarkable. At presentation, the patient exhibited depressed visual acuity on the left and defects of the temporal visual field. Ophthalmological examination found intact extraocular movements. Other neurological deficits were not noted. Cranial computed tomography scans revealed an isodense mass at the tip of the left middle fossa, 2 × 2 cm in maximal dimension, extending into the orbital apex, and accompanying bony erosions in the medial sphenoid ridge [
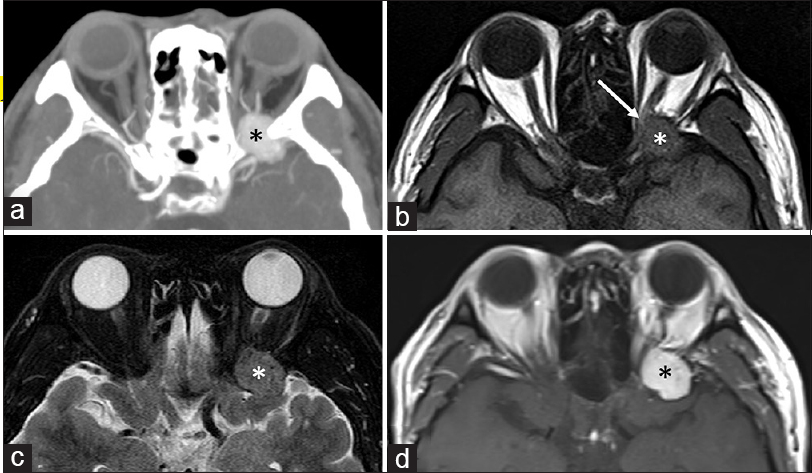
Figure 1
Axial computed tomography scan (a) and magnetic resonance images (b-d) showing a well-demarcated mass in the tip of the left temporal fossa extending into the orbital apex, and accompanying bony erosions in the medial sphenoid ridge (a). The tumor appears isointense on both T1- (b) and T2-weighted (c) sequences, and intensely enhanced on contrast examinations (a and d). The left optic nerve is considerably compressed by the tumour at the orbital apex (b, arrow)
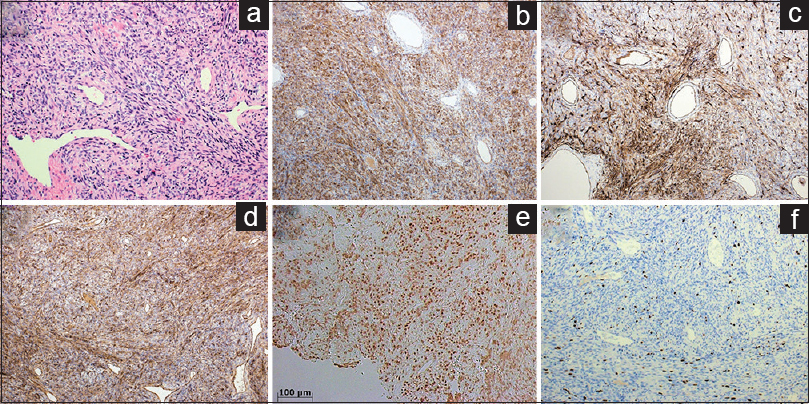
Figure 3
Microscopic appearance of the tumor comprised by spindle-shaped cells, lacking findings of atypia or necrosis (a). Immunohistochemical stains showing positive staining for bcl2 (b), CD34 (c), CD99 (d), and STAT6 (e), with the MIB-1 index of 10% (f). A: Hematoxylin and eosin stain, ×200; b: bcl2; c: CD34; d: CD99; e: STAT6; f: MIB-1, ×100
DISCUSSION
Based on the clinicopathological findings, the present case was thought to be a primary HP that originated from the middle fossa dura and grew as an epidural tumor. To our knowledge, this is the first report of a primary epidural HP originating in the cranial cavity. Intraoperatively, the meningo-orbital artery, a meningeal branch of the lacrimal or supraorbital arteries, connecting the orbit with the cranial cavity and passing through the orbitomeningeal foramina,[
The present HP considerably compressed the optic nerve predisposing to radiation injury. For the tumor, total resection was achieved. Meanwhile, the effectiveness of radiotherapy for HPs is not determined.[
HPs tend to cause local recurrence and distant metastases regardless of the histological grades.[
HP may present as an epidural tumor in the cranial cavity. HP should be assumed in the differential diagnosis during surgery of an intracranial epidural tumor. A total resection should be attempted when possible for such tumors.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Bruner JM, Tien RD, Enterline DS, Bigner DD, Mclendon RE, Bruner JM.editors. Tumors of the meninges and related tissues: Hemangiopericytomas of the meninges. Russel and Rubinstein’s pathology of tumors of the nervous system. London: Arnold; 1998. p. 112-7
2. Damodaran O, Robbins P, Knuckey N, Bynevelt M, Wong G, Lee G. Primary intracranial hemangiopericytoma: Comparison of survival outcomes and metastatic potential in WHO grade II and III variants. J Clin Neurosci. 2014. 21: 1310-4
3. Fountas KN, Kapsalaki E, Kassam M, Feltes CH, Dimopoulos VG, Robinson JS. Management of intracranial meningeal hemangiopericytomas: Outcome and experience. Neurosurg Rev. 2006. 29: 145-53
4. Ijiri K, Yuasa S, Yone K, Matsunaga S, Ryoki Y, Taniguchi N. Primary epidural hemangiopericytoma in the lumbar spine: A case report. Spine (Phila Pa 1976). 2002. 27: E189-92
5. Kaye AH, Laws ER.editorsBrain tumors. Tokyo: Livingstone; 1995. p. 705-11
6. Kumar N, Kumar R, Kapoor R, Ghoshal S, Kumar P, Salunke PS. Intracranial meningeal hemangiopericytoma: 10 years experience of a tertiary care Institute. Acta Neurochir (Wien). 2012. 154: 1647-51
7. Lin Z, Wang Y, Zhao M, Li Z, Chen X, Jiang Z. Unusual presentation of an intracranial hemangiopericytoma as a cystic intraparenchymal mass lesion closely mimicking a glioma. Neurol India. 2017. 65: 208-9
8. Macchi V, Regoli M, Bracco S, Nicoletti C, Morra A, Porzionato A. Clinical anatomy of the orbitomeningeal foramina: Variational anatomy of the canals connecting the orbit with the cranial cavity. Surg Radiol Anat. 2016. 38: 165-77
9. McDonald JV. Intraventricular hemangiopericytoma. J Neurosurg. 1999. 91: 167-
10. Melone AG, D'Elia A, Santoro F, Salvati M, Delfini R, Cantore G. Intracranial hemangiopericytoma-our experience in 30 years: A series of 43 cases and review of the literature. World Neurosurg. 2014. 81: 556-62
11. Olson C, Yen CP, Schlesinger D, Sheehan J. Radiosurgery for intracranial hemangiopericytomas: Outcomes after initial and repeat Gamma Knife surgery. J Neurosurg. 2010. 112: 133-9
12. Radley MG, McDonald JV. Meningeal hemangiopericytoma of the posterior fossa and thoracic spinal epidural space: Case report. Neurosurgery. 1992. 30: 446-52
13. Rutkowski MJ, Jian BJ, Bloch O, Chen C, Sughrue ME, Tihan T. Intracranial hemangiopericytoma: Clinical experience and treatment considerations in a modern series of 40 adult patients. Cancer. 2012. 118: 1628-36
14. Shetty PM, Moiyadi AV, Sridhar E. Primary CNS hemangiopericytoma presenting as an intraparenchymal mass-case report and review of literature. Clin Neurol Neurosurg. 2010. 112: 261-4
15. Stout AP, Murray MR. Hemangiopericytoma. Ann Surg. 1942. 116: 26-33
16. Towner JE, Johnson MD, Li YM. Intraventricular Hemangiopericytoma: A Case Report and Literature Review. World Neurosurg. 2016. 89: 728.e5-728.e10
17. Zeng L, Wang Y, Wang Y, Han L, Niu H, Zhang M. Analysis of prognosis-related factors of intracranial solitary fibrous tumors and hemangiopericytomas help understand the relationship between the two sorts of tumors. J Neurooncol. 2017. 131: 153-61
18. Zhu H, Duran D, Hua L, Tang H, Chen H, Zhong P. Prognostic Factors in Patients with Primary Hemangiopericytomas of the Central Nervous System: A Series of 103 Cases at a Single Institution. World Neurosurg. 2016. 90: 414-9