- Federal Centre of Treatment and Rehabilitation of Ministry of Healthcare of Russian Federation, Ivankovskoe, Moscow, Russia
Correspondence Address:
A. R. Sitnikov
Federal Centre of Treatment and Rehabilitation of Ministry of Healthcare of Russian Federation, Ivankovskoe, Moscow, Russia
DOI:10.4103/sni.sni_25_18
Copyright: © 2018 Surgical Neurology International This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.How to cite this article: A. R. Sitnikov, Yu A. Grigoryan, L. P. Mishnyakova. Bilateral stereotactic lesions and chronic stimulation of the anterior thalamic nuclei for treatment of pharmacoresistant epilepsy. 19-Jul-2018;9:137
How to cite this URL: A. R. Sitnikov, Yu A. Grigoryan, L. P. Mishnyakova. Bilateral stereotactic lesions and chronic stimulation of the anterior thalamic nuclei for treatment of pharmacoresistant epilepsy. 19-Jul-2018;9:137. Available from: http://surgicalneurologyint.com/surgicalint-articles/bilateral-stereotactic-lesions-and-chronic-stimulation-of-the-anterior-thalamic-nuclei-for-treatment-of-pharmacoresistant-epilepsy-2/
Abstract
Background:The use of the anterior nucleus of thalamus (ANT) as a target for treatment of pharmacoresistant epilepsy is based on its crucial role in seizure propagation. We describe results of chronic bilateral ANT stimulation and bilateral ANT lesions in 31 patients with refractory epilepsy.
Methods:ANT DBS was performed in 12 patients (group I) and bilateral stereotactic radiofrequency lesions of ANT were performed in 19 patients (group II). Targeting was based on stereotactic atlas information with correction of the final coordinates according to the location of anatomical landmarks and intraoperative microelectrode recording data.
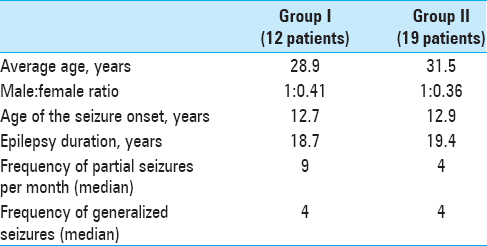
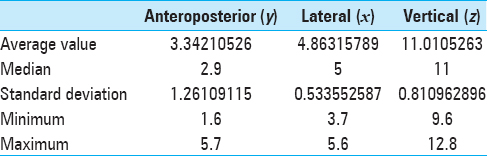
Results:Both groups were similar in age, gender, seizures frequency, and duration of disease. The median x, y, and z coordinates of ANT were found to be 2.9, 5, and 11 mm anterior, lateral, and superior to the mid-commissural point, respectively. Mean seizures reduction reached 80.3% in group of patients with ANT DBS with two nonresponders and 91.2% in group of patients with lesions. Five patients from group I and three patients from group II became seizure-free. The morbidity rate was low in both groups.
Conclusions:Stereotactic anterior thalamotomy and chronic ANT stimulation are both effective for seizure control in epilepsy originated from frontal and temporal lobes. ANT lesions and stimulation were more effective for secondary-generalized seizures compared to simple partial seizures.
Keywords: Anterior thalamic nucleus, deep brain stimulation, epilepsy, microelectrode recording seizures, stereotactic lesion
INTRODUCTION
Pharmacoresistant epilepsy can be defined as failure of adequate trials of two tolerated and appropriately chosen and used antiepileptic drugs schedules (whether as monotherapies or in combination) to achieve sustained seizure freedom.[
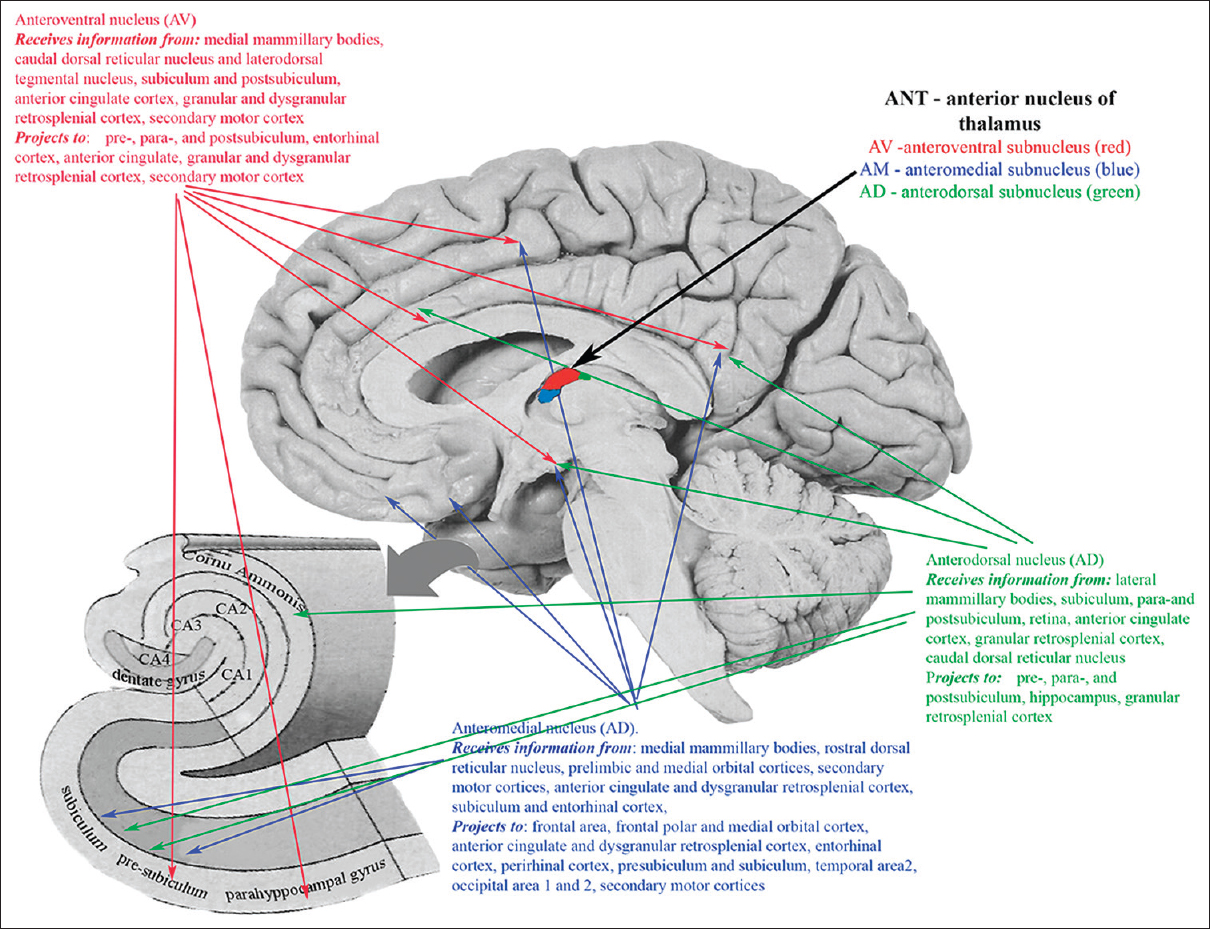
ANT represents a promising target because of their widespread projections to various cortical and subcortical structures and involvement in the process of generation and spreading of epileptic activity.[
ANT consist of 3 subnuclei (anteromedial nucleus, anterodorsal nucleus, and anteroventral nucleus) widely projecting predominantly to frontal and temporal lobes, including frontal area 2, frontal polar and medial orbital cortex, anterior cingulate and dysgranular retrosplenial cortex, entorhinal cortex, perirhinal cortex, presubiculum and subiculum, temporal area 2, and secondary motor cortex [
Various animal studies demonstrated the efficacy of bilateral lesions and stimulation of ANT in prevention of seizure propagation with superiority of lesions in terms of epilepsy control.[
The only research regarding the ANT lesions in humans was published by Mullan et al. in 1967. Using the strontium needle with exposition for 15 min he performed unilateral lesions in nine patients resulted in seizures-freedom in two patients, and seizures frequency reduction in four.[
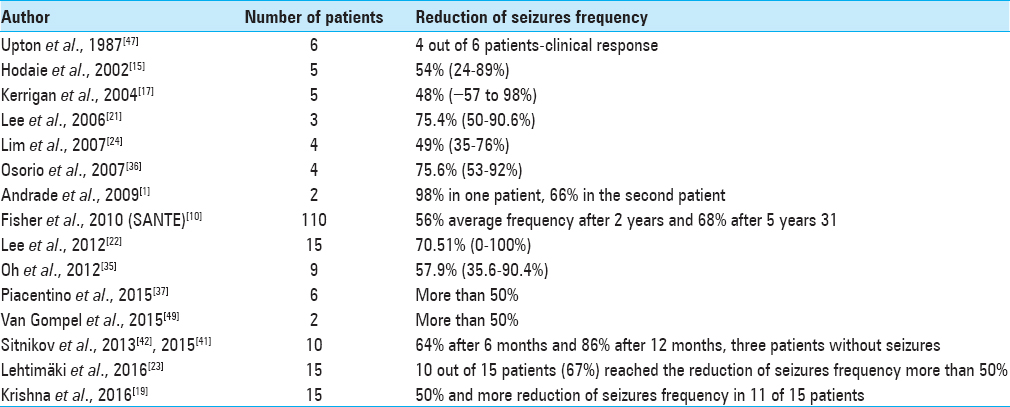
The article summarizes results of chronic electrical stimulation and bilateral radiofrequency lesion of the ANT in our series of patients with pharmacoresistant epilepsy.
MATERIALS AND METHODS
We operated on 31 patients with pharmacoresistant epilepsy (age 16–48 years). Twelve patients (group I) underwent bilateral ANT DBS and 19 patients (group II) underwent bilateral stereotactic radiofrequency ANT lesions. The major reasons for selecting patients for lesioning were as follows: 1) the patients’ disinclination to have the implantable devices, 2) the inability to come for reprogramming and stimulation settings checkup due to remote location from implanting center, 3) the inability to use the patients’ programmer due to intellectual decline.
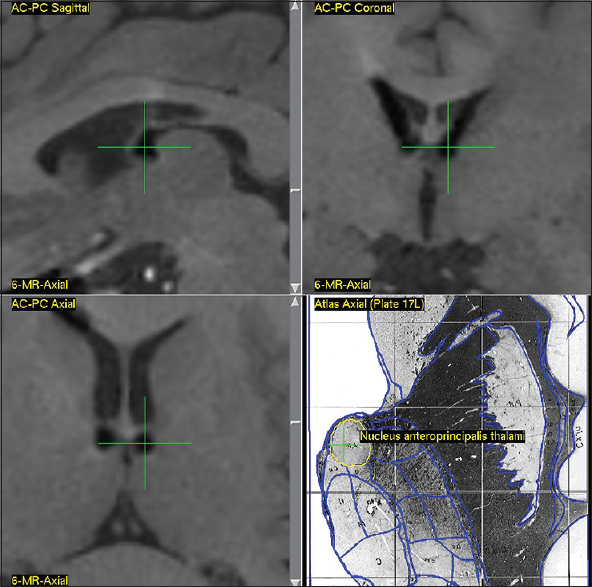
Due to lack of clearly identifiable anatomical borders of ANT on 3 Tesla MRI (GE Discovery MR750w), the patients were scanned day before surgery according to a standardized MR-protocol to visualize well-defined anatomical structures—subthalamic nucleus, red nucleus, and substantia nigra. The CT-scan with stereotactic frame (CRW® System, Integra™) was performed on the day of surgery using a routine protocol followed by the fusion of CT and MRI images. The combined stereotaxic targeting (anterior part of ANT) was performed using the Schaltenbrand and Wahren stereotaxic atlas with correction of final coordinates according to the deviation of visible anatomical targets (red nucleus, subthalamic nucleus) from the coordinates provided by atlas.
The procedure of implantation of electrodes or radiofrequency lesions was performed under a local anesthesia through bilateral 14 mm but holes located 3–3.5 cm posteriorly to the coronal suture and approximately 4 cm from midline. Microelectrode recording was performed in 11 patients from group I and in 18 patients in group II to differentiate the border between the lateral ventricle and the thalamus and define the length of ANT.
In 11 cases, four-contact electrodes (Medtronic 3389) were implanted and in one case eight-contact electrodes (Boston Scientific Vercise™ DBS system) were used.
For lesioning we used CSK-3M radiofrequency electrode (Cosman) with tip diameter 1.1 mm (the diameter of the insulated part of the electrode 1.24 mm, the length of the active tip is 3 mm) connected to G4 four-electrode RF generator (Cosman).
The position of electrodes after ANT DBS and location of lesions after bilateral thalamotomy were confirmed with MRI scan on the same day.
The results of surgical treatment were assessed with Engel and ILAE outcome scales, basing on the seizure diaries and control EEG.
RESULTS
Both groups of patients were comparable in average age, gender, duration of seizure history, and frequency of seizures [
In ANT DBS group, one patient diagnosed with idiopathic-generalized epilepsy, three had focal and eight had multifocal epilepsies. In ANT lesioning group, one patient had focal temporal epilepsy and 18-multifocal epilepsy with location of seizure onset zone(s) within frontal and/or temporal lobes, confirmed by continuous EEG-video monitoring.
Two of the patients in group I previously underwent resective surgery due to lesional epilepsy. One of them had focal cortical dysplasia resection (FCD) accompanied by multiple subpial transections in the right frontal lobe, resulting in incomplete seizure control for 2 years and subsequent increase in seizure frequency and severity. The second patient underwent incomplete FCD resection in the posterior part of the left temporal lobe with subsequent clinical and electroencephalographic remission and recurrence of symptoms 3 years later.
According to MRI data, structural abnormalities were noted in three patients from group II: in one patient posttraumatic gliotic changes in the left frontal, temporal, and parietal lobes, in one-transmantle FCD of the right parietal lobe, located in the eloquent area and one patient presented with multiple FCD in the left frontal, left temporal, and left insular lobes not eligible for surgical resection due to high risk of developing irreversible neurological deficit.
The estimated commissural coordinates of the targets (anterior thalamic nuclei) for implantation of stimulating electrodes and stereotaxic ATN lesion are presented in
The AP coordinates demonstrated the highest degree of variability due to the significant inter-hemispheric asymmetry of the foramen of Monroe and anatomy of the venous angle, formed by internal vein of the brain, thalamostriate vein, and the anterior septal vein, behind which is located the anterior tubercle of thalamus that contains ANT.
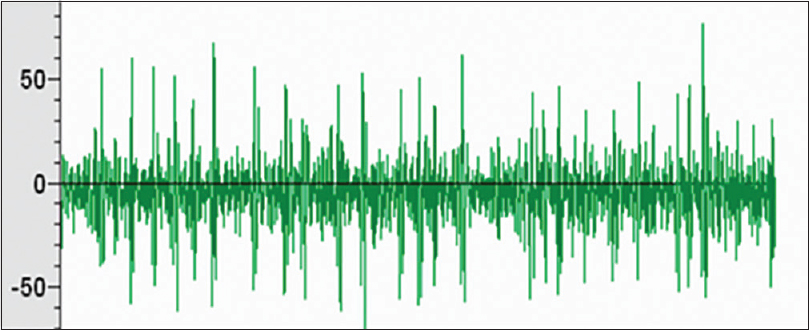
Microelectrode recording data were obtained from 56 trajectories; the clear signal from ANT was noted in 48 trajectories. The frequency of irregular high-amplitude spikes recorded when the microelectrode was passing ANT ranged from 15 to 29 Hz [
Group I
The postoperative MRI prior to IPG implantation confirmed correctness of bilateral electrode location in 11 patients [
Monopolar cathode stimulation started in the early postoperative period (2–3 days after surgery) with the initial parameters of 1.5 V, 110 Hz, 90 μs and subsequent adjustment of parameters based on clinical and EEG observation. At the time of hospital discharge, the average stimulation parameters were 4 V, 130 Hz, 90 μs.
Postoperative MRI revealed subcortical asymptomatic hematoma of the right frontal lobe with a volume of up to 3 cm3 located along the electrode track in one patient.
After implantation, all the patients went through adjustment of anticonvulsant therapy based on their seizure type and individual tolerance. Six patients were converted to monotherapy, six to a combination of two AEDs.
Follow-up evaluations and EEG—video monitoring were conducted in 3 months, with final correction of stimulation parameters at 6 months depending on its effectiveness.
There were no neurological side effects from stimulation. Two patients experienced “current leak” at the IPG site with monopolar mode stimulation; this phenomenon disappeared after the stimulation was changed to a bipolar mode.
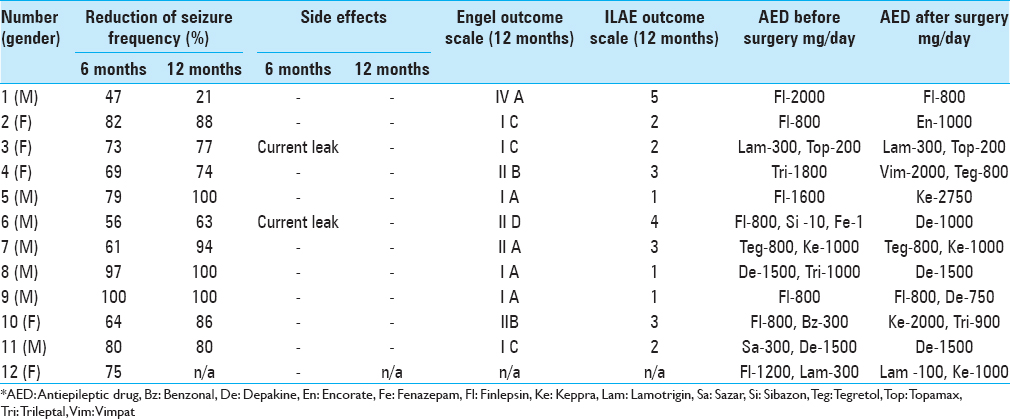
Follow-up ranged from 7 months to 5.2 years. The average reduction in seizure frequency a year after the start of stimulation was 80.3%, with three patients becoming seizure-free and four patients having only rare seizures (one of these patients has only night seizures), which are significantly lower in their duration and strength compared to preoperative period. One patient with positive MRI and extensive brain lesions did not respond to stimulation well [
One patient with a unilaterally displaced electrode failed to achieve significant improvement in terms of seizures; however the patient's quality of life improved as a result of objective improvement in cognitive and psychoemotional status.
One patient had an infection along his extension cables resulted in explantation of IPG and the extension cables lines with the preservation of intracranial electrodes.
Group II
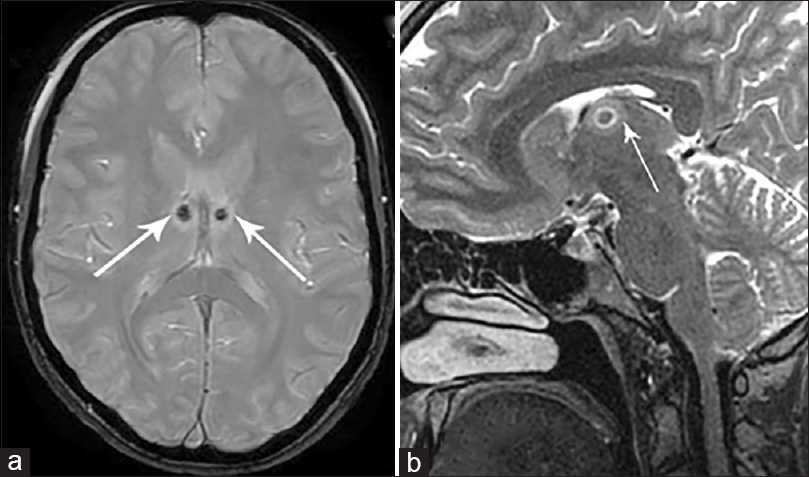
In group II patients, postoperative MRI confirmed the location of the lesions zones within ANT on both sides with average diameter of the lesion zone 4.1 mm (range 3.8–5.2 mm) [
In one patient the postoperative MRI scan revealed noticeable lesion only on one side due to technical mistake (mismeasurement of RF-electrode length) requiring repeated unilateral radiofrequency lesion the next day with successfully placed lesion within ANT borders.
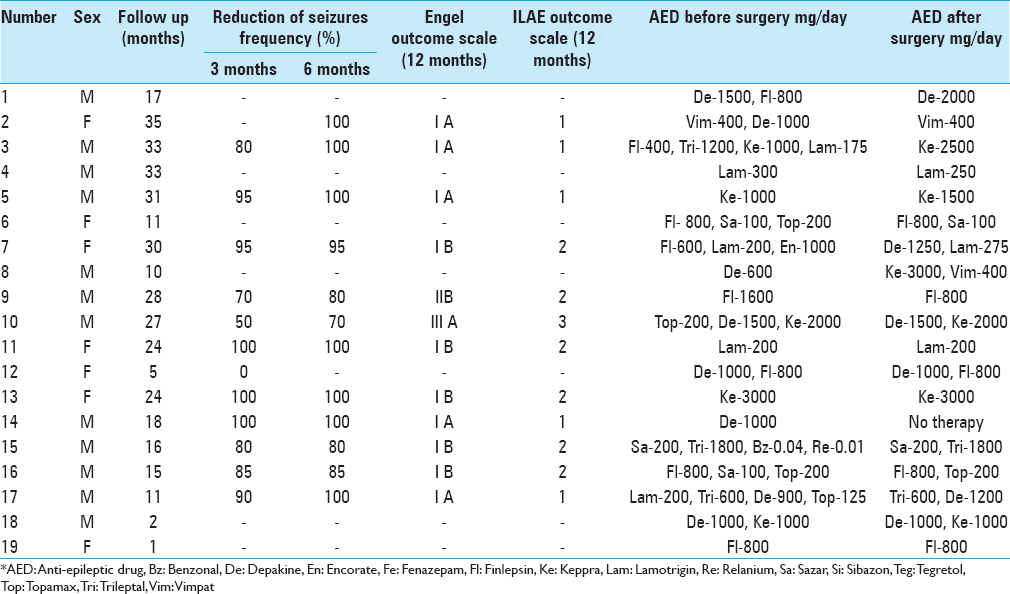
During early postoperative period, 16 of the 19 patients experienced a significant reduction in seizures ranging from 50% to 90%. Two patients with a previously registered photoparoxysmal response demonstrated increased resistance to photic stimulaiton. In one case, the EEG obtained 12 months after the surgery did not show any pathological activity [
The average seizure frequency reduction among the responders with available follow-up exceeded 91.2%. Currently, five patients remain completely seizure-free.
The postoperative period was uneventful in all patients except one who developed a small right-sided subcortical hematoma and transient left-side hemiparesis that resolved in 4 weeks.
No side effects or any significant changes in mental and emotional status were observed after the lesions.
DISCUSSION
Stimulation of the anterior thalamic nuclei for the treatment of epilepsy was first performed in 1980s by Cooper I.S. and Upton A.R. and soon thereafter by Sussman N.M.[
In most studies, the authors observed effective suppression of the seizures frequency using high-frequency stimulation in a cyclic mode [
The exact mechanism of action of ANT DBS remains poorly understood. First, the explanation of mechanism of action has to be thought as a result of inactivation of stimulated structures due to the similarity in therapeutic effects with DBS and lesions. However, the possible mechanism of action seems to be more complicated. Model-based analysis of the cellular effects of DBS provided by McIntyre C.C. et al. revealed both excitatory and inhibitory effects on the thalamic neurons located near the electrode.[
Griffin D. M. et al. and later Cheney P. D. et al. described the mechanism of “neural hijacking” during intracortical microstimulation and recording the action potentials from single neurons.[
Other thalamic nuclei also have been proposed for DBS in refractory epilepsy. Velasco F. et al. reported five cases of centro-median thalamic nucleus (CMT) DBS in 1987 showing the significant reduction in seizures frequency.[
Fisher R. S. et al. in 1992 also reported at least a 50% decrease in seizure frequency in three of six patients with CMT DBS with no side effects.[
More recent study published by Valentín A. in 2013 included 11 patients (five with frontal lobe epilepsy, six with primary-generalized epilepsy) treated with CMT DBS.[
All published data regarding to CMT DBS clearly showing the great efficacy for primary-generalized seizures, especially in cases of Lennox–Gastaut syndrome with less prominent effectiveness in cases of frontal or temporal lobe partial epilepsy in contrast with ANT DBS that is more suitable for cases of secondary-generalized seizures originated from temporal/frontal lobes.
The parameters of ANT stimulation still remain empirical. In the SANTE trial the stimulation with 5V 145Hz 90 μs in cycling mode (1 min on/5 min off) resulted in 76% median reduction in seizure frequency at 5 years.[
Animal study provided by Gibson W. S. et al. showed an amplitude-dependent increase activation within temporal, prefrontal, and sensorimotor cortex in at 60 Hz and 145 Hz stimulation, but not with 2 Hz stimulation.[
The use of various electrodes and modes for stimulation also can be an issue. In most studies the majority of patients were implanted with Medtronic 3387 leads following by monopolar stimulation. Patients in our study were implanted with Medtronic 3389 leads (one case eight-Boston Scientific Vercise™ DBS electrodes) with shorter inter-contact spacing (0.5 mm) and were stimulated with monopolar configuration. The use of electrodes with shorter spacing more likely allows to implant two contacts within ANT and change parameters to bipolar mode if needed without current spreading on adjacent thalamic nuclei. In two patients from our group who experienced current leak with monopolar stimulation, we achieved the parameters correction from monopolar to bipolar configuration without reducing the amplitude/frequency and with the same efficacy in seizures reduction.
The issue of stereotactic planning in the anterior thalamic nuclei is extremely important, because effective seizure frequency reduction is possible only with bilateral ANT stimulation or lesion.[
Möttönen T., Lehtimäki K. et al. demonstrated the possibility of a precise delineation of the ANT anatomical boundaries using STIR and T1-MPRAGE MR-sequences.[
Recently, Krishna V. et al. showed that microelectrode recording can be used to identify the nature of the signal from the ANT, however it is not mandatory due the signal nonspecificity.[
The characteristic data of the ANT signal and anterior ventral thalamic nuclei (VA) (three spike per 2 s for ANT and seven spikes per 2 s for VA), shown in work of Lehtimäki et al., are similar to what we saw in our cases and confirm the theory that firing rate is more specific for ANT than the signal shape and/or interspike interval.[
Due to presumably higher risk of hemorrhagic complications the use of microelectrode recording in ANT DBS still controversial, especially considering the transventricular trajectory of electrode placement. However, recent data showing the minimal, if not a zero risk of microelectrode recording-associated hemorrhages.[
Another technical complication noted in patients with ANT DBS was unilateral misplacement of DBS electrode. The possibility of electrode misplacement during ANT DBS also was specified by other studies, resulted in recommendation of obligatory postoperative MRI to confirm the electrodes location, irrespective of neurophysiological conformation and preoperative targeting.[
Stereotactic lesioning of anterior thalamus predates ANT DBS by about 20 years. The first report on unilateral stereotactic lesion of the anterior thalamus in humans for the treatment of epileptic seizures was published in 1967 by Mullan S. et al.[
With modern MRI and targeting techniques the precise placement of lesions gives the alternative to ANT DBS in some selected cases as has been shown in our study. The same efficacy of bilateral ANT stimulation and lesions in terms of seizures control confirm the major role of ANT in propagation of epileptic activity originated from temporal/frontal lobes. However, more studies needed to specify the indications to both procedures and to define best candidates for ANT DBS/ANT lesions among patients suffering from medically intractable seizures.
CONCLUSION
Stereotactic radiofrequency anterior nucleotalamotomy and high-frequency ANT stimulation appear to have similar efficacy in terms of seizures control in patients with pharmacoresistant epilepsy with single or multiple sources of pathological activity located in the frontal and temporal lobes. The maximum efficiency of both approaches is observed in the suppression of secondarily generalized seizures, whereas simple partial seizures are less responsive.
Use of microelectrode recording facilitates accurate determination of neurophysiological borders of the anterior thalamic nuclei and appears to improve surgical treatment results.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Andrade DM, Hamani C, Lozano AM, Wennberg RA. Dravet syndrome and deep brain stimulation: seizure control after 10 years of treatment. Epilepsia. 2009. 51: 1314-6
2. Benabid AL, Koudsie A, Benazzouz A, Vercueil L, Fraix V, Chabardes S. Deep brain stimulation of the corpus Luysi (subthalamic nucleus) and other targets in Parkinson's disease. Extension to new indications such as dystonia and epilepsy. J Neurol. 2001. 248: 37-47
3. Buentjen L, Kopitzki K, Schmitt FC, Voges J, Tempelmann C, Kaufmann J. Direct targeting of the thalamic anteroventral nucleus for deep brain stimulation by T1-weighted magnetic resonance imaging at 3 T. Stereotact Funct Neurosurg. 2014. 92: 25-30
4. Cheney PD, Griffin DM, Van Acker GM. Neural hijacking: Action of high-frequency electrical stimulation on cortical circuits. Neuroscientist. 2013. 19: 434-41
5. Child ND, Benarroch EE. Anterior nucleus of the thalamus: Functional organization and clinical implications. Neurology. 2013. 81: 1869-76
6. Chou YC, Lin SZ, Hsieh WA, Lin SH, Lee CC, Hsin YL. Surgical and hardware complications in subthalamic nucleus deep brain stimulation. J Clin Neurosci. 2007. 14: 643-9
7. Cooper IS, Upton AR. Therapeutic implications of modulation of metabolism and functional activity of cerebral cortex by chronic stimulation of cerebellum and thalamus. BiolPsychiatry. 1985. 20: 811-3
8. Cossu M, Fuschillo D, Casaceli G, Pelliccia V, Castana L, Mai R. Stereoelectroencephalography-guided radiofrequency thermocoagulation in the epileptogenic zone: Aretrospective study on 89 cases. J Neurosurg. 2015. 123: 1358-67
9. Falowski S, Dierkes J. An analysis of the use of multichannel microelectrode recording during deep brain stimulation surgeries at a single center. Oper Neurosurg. 2017. 0: 1-8
10. Fisher R, Salanova V, Witt T, Worth R, Henry T, Gross R. Electrical stimulation of the anterior nucleus of thalamus for treatment of refractory epilepsy. Epilepsia. 2010. 51: 899-908
11. Fisher RS, Uematsu S, Krauss GL, Cysyk BJ, McPherson R, Lesser RP. Placebo-controlled pilot study of centromedian thalamic stimulation in treatment of intractable seizures. Epilepsia. 1992. 33: 841-851
12. Gibson WS, Ross EK, Han SR, Van Gompel JJ, Min H.K., Lee KH. Anterior thalamic deep brain stimulation: Functional activation patterns in a large animal model. Brain Stimul. 2016. 9: 770-3
13. Griffin DM, Hudson HM, Belhaj-Saif A, Cheney PD. Hijacking cortical motor output with repetitive microstimulation. J Neurosci. 2011. 31: 13088-96
14. Hamani C, Ewerton FI, Bonilha SM, Ballester G, Mello LE, Lozano AM. Bilateral anterior thalamic nucleus lesions and high-frequency stimulation are protective against pilocarpine-induced seizures and status epilepticus. Neurosurgery. 2004. 54: 191-5
15. Hodaie M, Wennberg RA, Dostrovsky JO, Lozano AM. Chronic anterior thalamus stimulation for intractable epilepsy. Epilepsia. 2002. 43: 603-8
16. Jiltsova E, Möttönen T, Fahlström M, Haapasalo J, Tähtinen T, Peltola J. Imaging of anterior nucleus of thalamus using 1.5T MRI for deep brain stimulation targeting in refractory epilepsy. Neuromodulation. 2016. 19: 812-7
17. Kerrigan JF, Litt B, Fisher RS, Cranstoun S, French JA, Blum DE. Electrical stimulation of the anterior nucleus of the thalamus for the treatment of intractable epilepsy. Epilepsia. 2004. 45: 346-54
18. Kim SH, Son BC, Lim SC, Kim WJ, Bae DW, Shon YM. EEG driving response during low-frequency stimulation of anterior thalamic nucleus: Is it a good predictor of the correct location of DBS electrode?. Clin Neurophysiol. 2014. 125: 1065-6
19. Krishna V, Kon KKN, Sammartino F, Strauss I, Andrade DM, Wennberg RA. Anterior nucleus deep brain stimulation for refractory epilepsy insights into patterns of seizure control and efficacious target. Neurosurgery. 2016. 78: 802-11
20. Kwan P, Arzimanoglou A, Berg AT, Brodie MJ, Allen Hauser W, Mathern G. Definition of drug resistant epilepsy: Consensus proposal by the ad hoc Task Force of the ILAE Commission on Therapeutic Strategies. Epilepsia. 2010. 51: 1069-77
21. Lee KJ, Jang KS, Shon YM. Chronic deep brain stimulation of subthalamic and anterior thalamic nuclei for controlling refractory partial epilepsy. Acta Neurochir. 2006. p. 87-91
22. Lee KJ, Shon YM, Cho CB. Long-term outcome of anterior thalamic nucleus stimulation for intractable epilepsy. Stereotactic Funct Neurosurg. 2012. 90: 379-85
23. Lehtimäki K, Möttönen T, Järventausta K, Katisko J, Tähtinen T, Haapasalo J. Outcome based definition of the anterior thalamic deep brain stimulation target in refractory epilepsy. Brain Stimul. 2016. 9: 268-75
24. Lim SN, Lee ST, Tsai YT, Chen IA, Tu PH, Chen JL. Long-term anterior thalamus stimulation for intractable epilepsy. Chang Gung Med J. 2008. 31: 287-96
25. Luo H, Zhao Q, Tian Z, Wu Z, Wang F, Lin H. Bilateral stereotactic radiofrequency amygdalohippocampectomy for a patient with bilateral temporal lobe epilepsy. Epilepsia. 2013. 54: 155-8
26. Malikova H, Kramska L, Vojtech Z, Sroubek J, Lukavsky J, Liscak R. Relationship between remnant hippocampus and amygdala and memory outcomes after stereotactic surgery for mesial temporal lobe epilepsy. Neuropsychiatr Dis Treat. 2015. 11: 2927-33
27. McIntyre CC, Grill WM, Sherman DL, Thakor NV. Cellular effects of deep brain stimulation: Model-based analysis of activation and inhibition. JNeurophysiol. 2004. 91: 1457-69
28. Mirski MA, Ferrendelli JA. Anterior thalamic mediation of generalized pentylenetetrazol seizures. Brain Res. 1986. 399: 212-23
29. Mirski MA, Ferrendelli JA. Interruption of the mammillothalamic tract prevents seizures in guinea pigs. Science. 1984. 226: 72-4
30. Mirski MA, McKeon AC, Ferrendelli JA. Anterior thalamus and substantia nigra: Rwo distinct structures mediating experimental generalized seizures. Brain Res. 1986. 397: 377-80
31. Mondragon S, Lamarche M. Suppression of motor seizures after specific thalamotomy in chronic epileptic monkeys. Epilepsy Res. 1990. 5: 137-45
32. Morgan VL, Rogers BP, Abou-Khalil B. Segmentation of the thalamus based on BOLD frequencies affected in temporal lobe epilepsy. Epilepsia. 2015. 56: 1819-27
33. Möttönen T, Katisko J, Haapasalo J, Tähtinen T, Saastamoinen A, Peltola J. The correlation between intraoperative microelectrode recording and 3-Tesla MRI in patients undergoing ANT-DBS for refractory epilepsy. Stereotact Funct Neurosurg. 2016. 94: 86-92
34. Mullan S, Vailati G, Karasick J, Mailis M. Thalamic lesions for the control of epilepsy: Astudy of nine cases. Arch Neurol. 1967. 16: 277-85
35. Oh YS, Kim HJ, Lee KJ, Kim YI, Lim SC, Shon YM. Cognitive improvement after long-term electrical stimulation of bilateral anterior thalamic nucleus in refractory epilepsy patients. Seizure. 2012. 21: 183-7
36. Osorio I, Overman J, Giftakis J, Wilkinson SB. High frequency thalamic stimulation for inoperable mesial temporal epilepsy. Epilepsia. 2007. 48: 1561-71
37. Piacentino M, Durisotti C, Garofalo PG, Bonanni P, Volzone A, Ranzato F. Anterior thalamic nucleus deep brain stimulation (DBS) for drug-resistant complex partial seizures (CPS) with or without generalization: Long-term evaluation and predictive outcome. Acta Neurochir. 2015. 157: 1525-32
38. Salanova V, Witt T, Worth R, Henry TR, Gross RE, Nazzaro JM. Long-term efficacy and safety of thalamic stimulation for drug-resistant partial epilepsy. Neurology. 2015. 84: 1017-25
39. Sansur CA, Frysinger RC, Pouratian N, Fu KM, Bittl M, Oskouian RJ. Incidence of symptomatic hemorrhage after stereotactic electrode placement. J Neurosurg. 2007. 107: 998-1003
40. Sitnikov AR, Grigoryan YuA, Mishnyakova LP. Bilateral radiofrequency anterior thalamotomy in patients with pharmacoresistant epilepsy. Zh Vopr Neirokhir Im NN Burdenko. 2016. 80: 25-34
41. Sitnikov AR, Grigoryan YuA, Mishnyakova LP. Stimulation of the anterior thalamic nuclei of intraoperative microelectrode recording in treatment of pharmacoresistant epilepsy. Russian neurosurgical journal named after Professor A. L. Polenov. 2015. VII: 61-69
42. Sitnikov AR, Grigoryan YuA, Mishnyakova LP, Vlasova RM. Chronic stimulation of the anterior nuclei of the thalamus in pharmacoresistant epilepsy. Russian neurosurgical journal named after Professor A.L. Polenov. 2013. 5: 27-33
43. Son B, Shon YM, Kim S, Choi J, Kim J. Relationship between postoperative EEG driving response and lead location in deep brain stimulation of the anterior nucleus of the thalamus for refractory epilepsy. Stereotact Funct Neurosurg. 2016. 94: 336-41
44. Sussman NM, Goldman HW, Jackel RA, Kaplan L, Callanan M, Bergen J. Anterior thalamus stimulation in medically intractable epilepsy, part II: preliminary Preliminary clinical results. Epilepsia. 1988. 29: 677-
45. Sweeney-Reed CM, Lee H, Rampp S, Zaehle T, Buentjen L, Voges J. Thalamic interictal epileptiform discharges in deep brain stimulated epilepsy patients. J Neurol. 2016. 263: 2120-6
46. Upton AR, Amin I, Garnett S, Springman M, Nahmias C, Cooper IS. Evoked metabolic responses in the limbic striate system produced by stimulation of anterior thalamic nucleus in man. Pacing Clan Electrophysiol. 1987. 10: 217-25
47. Upton AR, Cooper IS, Springman M, Amin I. Suppression of seizures and psychosis of limbic system origin by chronic stimulation of anterior nucleus of the thalamus. Int J Neurol. 1985-1986. 19-20: 223-30
48. Valentín A, García Navarrete E, Chelvarajah R, Torres C, Navas M, Vico L. Deep brain stimulation of the centromedian thalamic nucleus for the treatment of generalized and frontal epilepsies. Epilepsia. 2013. 54: 1823-33
49. Van Gompel JJ, Klassen BT, Worrell GA, Lee KH, Shin C, Zhao CZ. Anterior nuclear deep brain stimulation guided by concordant hippocampal recording. Neurosurg Focus. 2015. 38: E9-
50. Velasco AL, Velasco F, Jiménez F, Velasco M, Castro G, Carrillo-Ruiz JD. Neuromodulation of the centromedian thalamic nuclei in the treatment of generalized seizures and the improvement of the quality of life in patients with Lennox–Gastaut syndrome. Epilepsia. 2006. 47: 1203-12
51. Velasco F, Velasco M, Ogarrio C, Fanghanel G. Electrical stimulation of the centromedian thalamic nucleus in the treatment of convulsive seizures: Apreliminary report. Epilepsia. 1987. 28: 421-30
52. Velasco F, Velasco M, Velasco AL, Jimenez F. Effect of chronic electrical stimulation of the centromedian thalamic nuclei on various intractable seizure patterns: I. Clinical seizures and paroxysmal EEG activity. Epilepsia. 1993. 34: 1052-64
53. Velasco F, Velasco M, Jimenez F, Velasco AL, Marquez I. Stimulation of the central median thalamic nucleus for epilepsy. Stereotact Funct Neurosurg. 2001. 77: 228-32
54. Velasco M, Velasco F, Velasco AL, Velasco G, Jiménez F. Effect of chronic electrical stimulation of the centromedian thalamic nuclei on various intractable seizure patterns: II. Psychological performance and background EEG activity. Epilepsia. 1993. 34: 1065-74
55. . https://www.fda.gov/default.htm [homepage on the Internet].
56. Wright NF, Vann SD, Erichsen JT, O’Mara S, Aggleton JP. Segregation of parallel inputs to the anteromedial and anteroventral thalamic nuclei of the rat. J Comp Neurol. 2013. 521: 2966-86