- Department of Neurosurgery, Riverside University Health Systems, Moreno Valley, USA
- Department of Neurosurgery, Columbia University College of Physicians and Surgeons, New York, USA
- Department of Neurosurgery, University of California Los Angeles, Los Angeles, USA
- Department of Neurosurgery, Stanford University School of Medicine, Stanford, USA
- Department of Neurological Surgery, Kaiser Permanente Orange County, Anaheim, USA
- Department of Obstetrics and Gynecology, University of California Los Angeles Medical Center, Santa Monica, USA
- Department of Medicine, Shahid Sadoughi University of Medical Sciences and Health Services, Yazd, Yazd Province, Iran.
- Department of Internal Medicine, Center for Medical Education and Research, University of California San Francisco, Fresno, USA
- Department of Neurology, Harvard Medical School, Boston, MA, USA
- 0Department of Oncology, Stanford Cancer Center, Stanford University School of Medicine, Stanford, CA, USA,
Correspondence Address:
Ariel Takayanagi
0Department of Oncology, Stanford Cancer Center, Stanford University School of Medicine, Stanford, CA, USA,
DOI:10.25259/SNI_37_2019
Copyright: © 2019 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Ariel Takayanagi, T. J. Florence, Omid R. Hariri, Abigail Armstrong, Pouria Yazdian, Andrew Sumida, Syed A. Quadri, Joshua Cohen, Omid S. Tehrani. Brain metastases from cervical cancer reduce longevity independent of overall tumor burden. 13-Sep-2019;10:176
How to cite this URL: Ariel Takayanagi, T. J. Florence, Omid R. Hariri, Abigail Armstrong, Pouria Yazdian, Andrew Sumida, Syed A. Quadri, Joshua Cohen, Omid S. Tehrani. Brain metastases from cervical cancer reduce longevity independent of overall tumor burden. 13-Sep-2019;10:176. Available from: http://surgicalneurologyint.com/surgicalint-articles/brain-metastases-from-cervical-cancer-reduce-longevity-independent-of-overall-tumor-burden/
Abstract
Background: Isolated brain metastasis (IBM) from cervical cancer is a very rare encounter in neurosurgery. We sought to understand how patients with isolated brain metastases differ from those with metastases in the setting of widespread disease.
Methods: A systematic review was completed using PubMed and the Cochrane Library. Patients with isolated brain metastases (IBM) and non-isolated brain metastases (NIBM, or brain metastases in the setting of disseminated disease), were compared. Two-sided statistical tests were used to determine significance. Survival function was carried out using the Kaplan–Meier method.
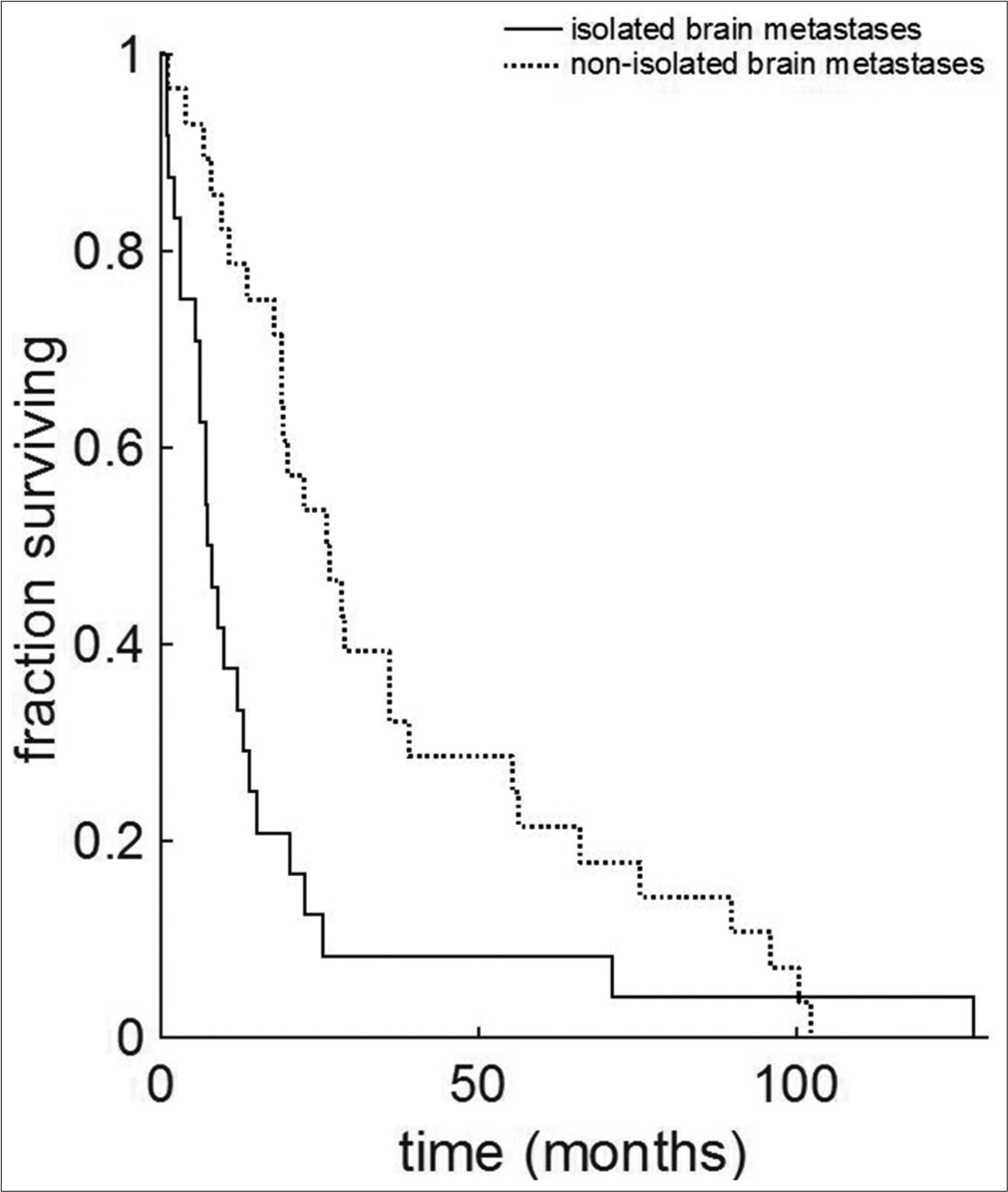
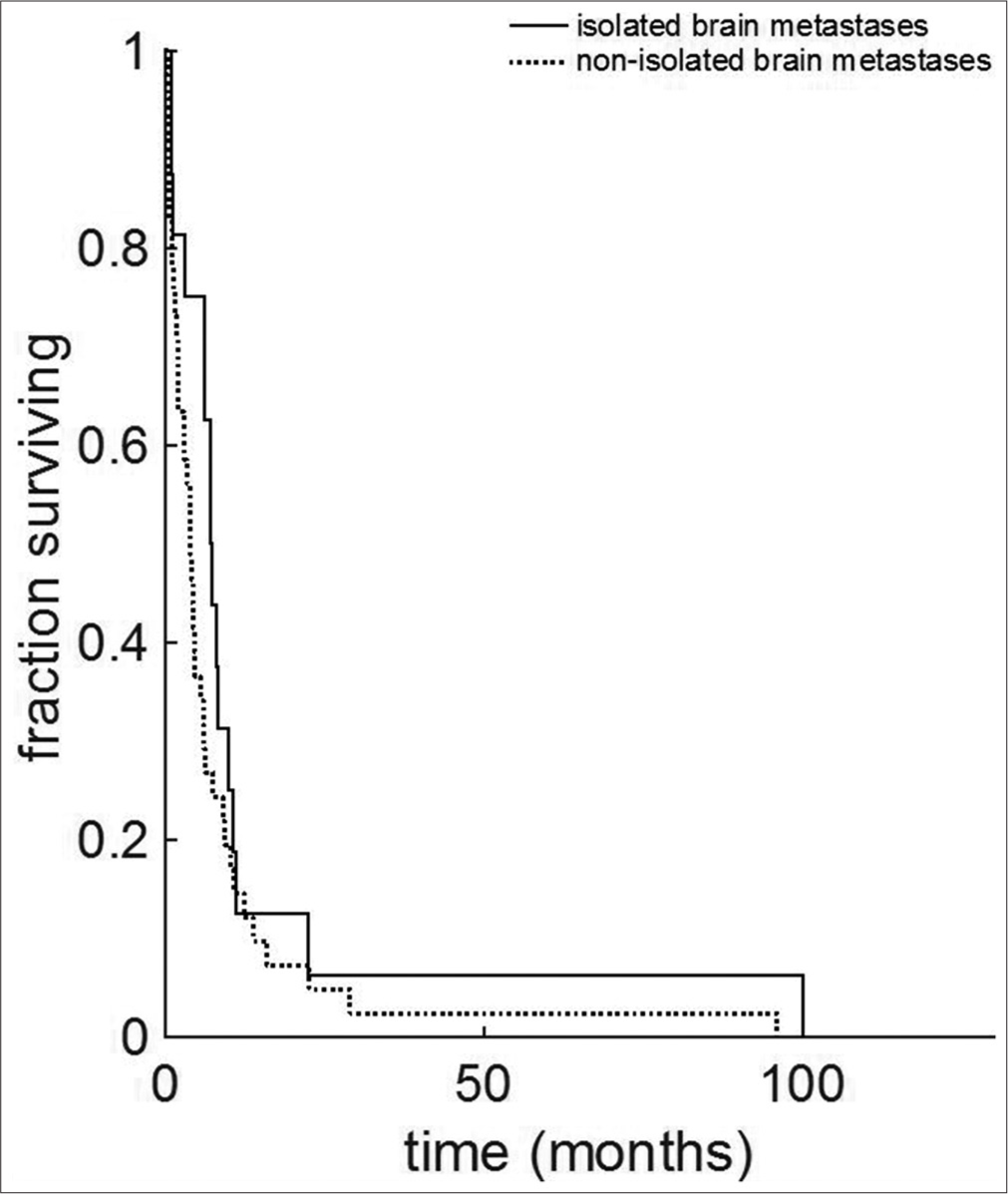
Results: A total of 89 patients, 25 with IBM and 64 with NIBM, were identified. The time interval between initial diagnosis of cervical cancer and diagnosis of brain lesion was significantly shorter in the IBM group (median 7.5 vs. 20.05 months, and IBM vs. NIBM, respectively; P = 0.006). Overall survival from initial diagnosis of cervical cancer was significantly shorter for the IBM group versus the NIBM group (7.63 vs. 26.3 months, respectively; P = 0.0005). Data demonstrate a 3.4-fold reduction of median life expectancy to 7.63 months. Survival after diagnosis of brain metastases did not differ between groups (median, IBM 7 months vs. NIBM 4 months, P = 0.08).
Conclusion: Taken together, our data suggest that for cervical cancer patients with brain metastasis intracranial metastasis itself (and not overall tumor burden) represent a sentinel event in limiting longevity. While the present study is underpowered to compare treatment options directly, further work should be focused on determining the optimal treatment for these patients.
Keywords: Brain, Cancer, Cervical, Cervix, Isolated, Metastases, Non-isolated, Survival, Uterine, Uterus
INTRODUCTION
Cervical cancer is an aggressive gynecological cancer of the uterine cervix. Tumors may consist of one of many histopathologies, from the more common squamous or adenocarcinomatous tumors to less common neuroendocrine tumors. While incidence in the US has decreased due to the widespread screening of cervical cytology and adoption of the human papillomavirus vaccine, prevalence remains at a value of roughly 6.8 cases per 100,000 women per year.[
Brain metastasis represents a rather uncommon but known complication. The estimated frequency of brain metastases from cervical cancer seen in the clinical setting ranges from 0.4% to 2%, while autopsy studies have reported brain metastases in 3–10% of cervical cancer patients.[
It remains unclear if IBM represents a distinct clinical entity from NIBM. Previous reports[
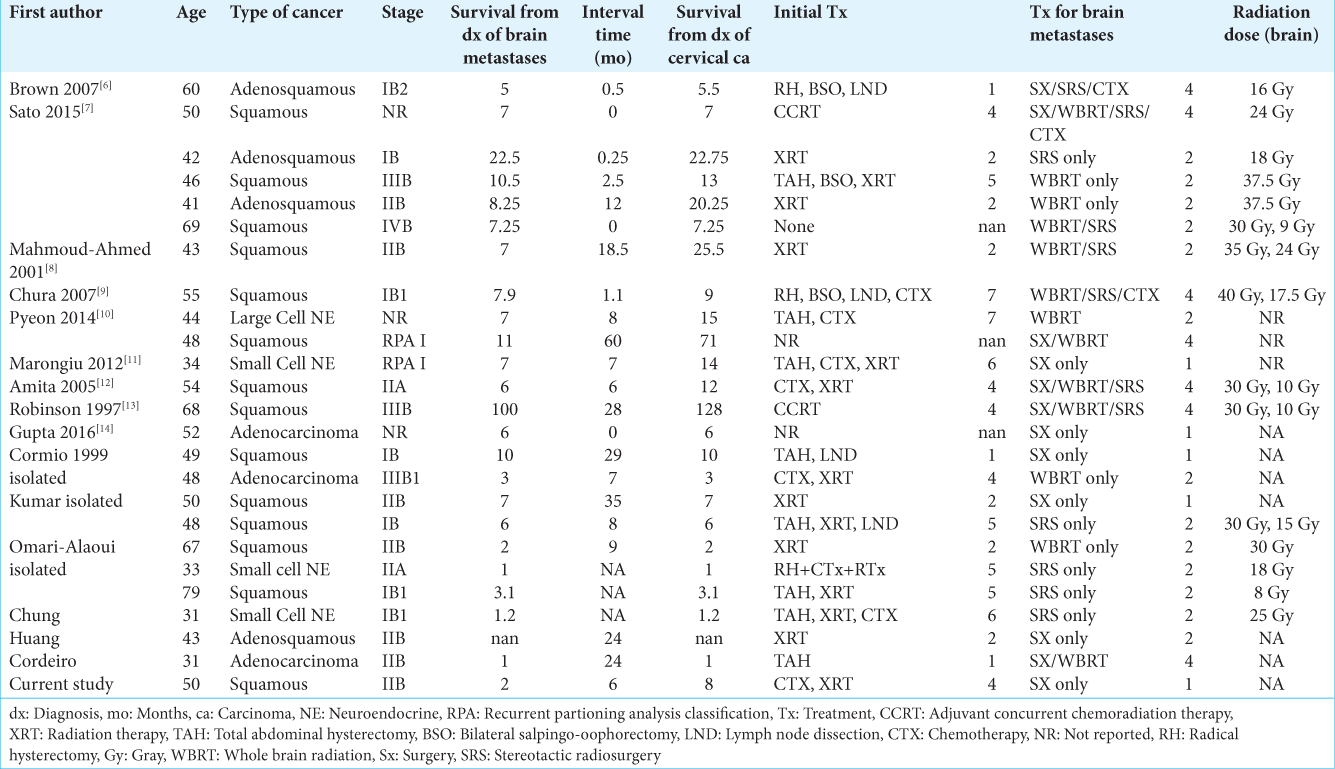
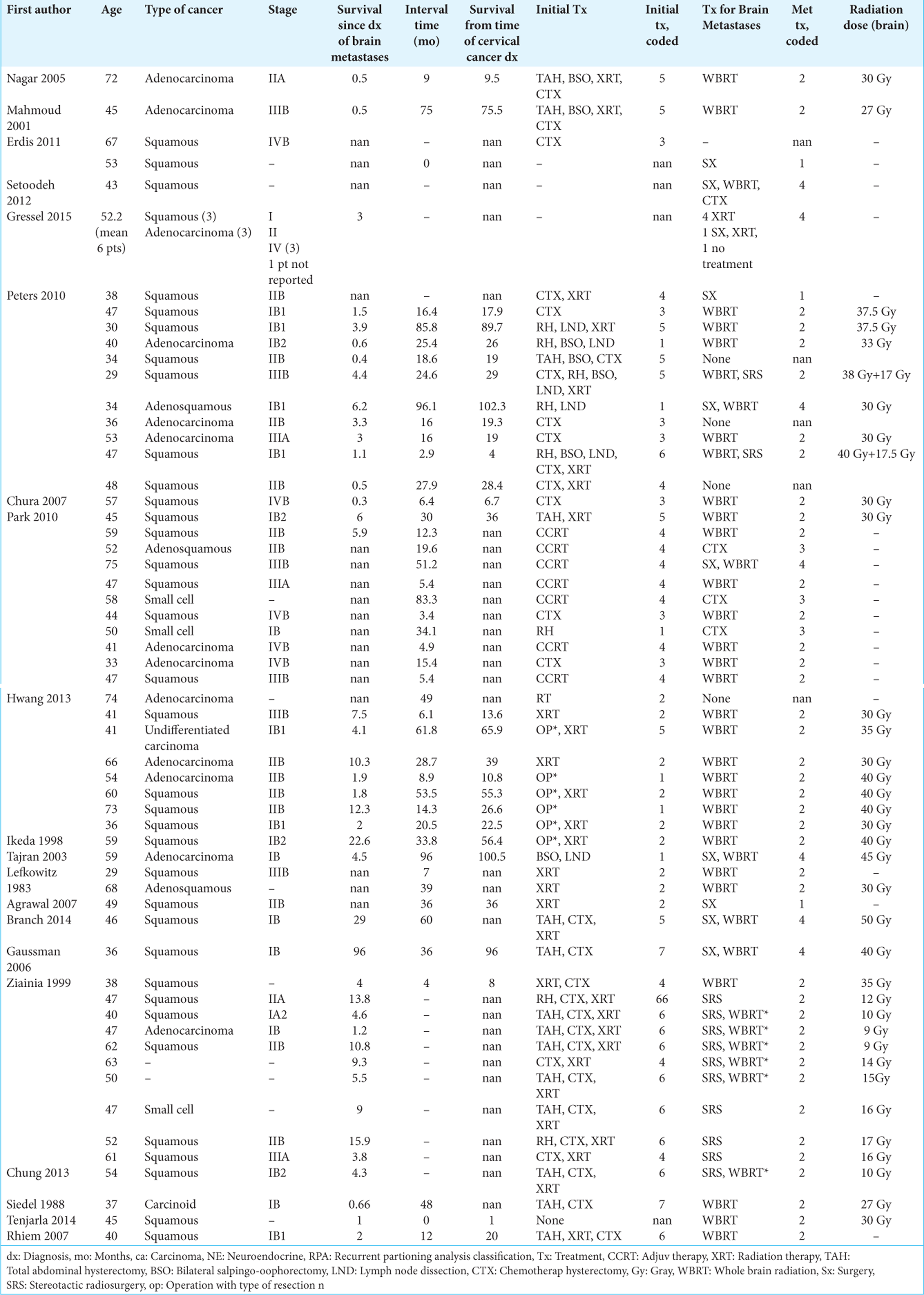
This systematic review serves to determine possibly identify differences by comparing 25 cases of IBM to 64 cases of NIBM. The 25 cases consist of 24 cases found in the literature, and one case that presented to our institution. Although other cases of IBM and NIBM from cervical cancer have been reported, many reports neglect to report key details about the cases and could not be included in this study.
MATERIALS AND METHODS
A review of the published literature before August 2018 was conducted using biomedical databases PubMed, OVID, Medline, Web of Knowledge, and EMBASE. We sought peer- reviewed articles on brain metastasis. Terms for the search included “brain metastases,” “isolated brain metastases,” “cervical cancer metastases,” “cervical cancer brain metastases,” and “uterine cervical cancer brain metastases.” The search was temporally restricted to 35 years, between 1983 and 2018, to ensure cases contained those followed with modern computed tomography scanning. Exclusion criteria were directed at removing low-quality case reports and case series, operationally defined as publications not meeting 8/10 of Joanna Briggs Institute (JBI) criteria for case series, or 6/8 JBI criteria for case reports (as applicable). PRISMA guidelines were followed for reporting the qualitative results. The decision to involve or eliminate all relevant articles and data extraction was completed by the authors, and any controversies and disagreements were settled by discussion.
Inclusion criteria
All studies with one or more cases of cervical cancer with brain metastases with details specific to each patient were included, such as histology, stage, survival, and treatment.
Exclusion criteria
Articles presenting at autopsy, in vitro studies, and any animal studies were excluded from the study. In addition, articles were excluded if the extent of metastases (isolated to brain versus systemic) was not specified. Cases without details of survival were not included in our analysis. Further, duplicate articles in these databases and full-text articles not written in English were also screened and excluded. Similarly, opinion letters, short reviews, very old case reports, and studies with the possibility of blurred/mixed and confusing data were excluded from the study.
Data extraction
The following characteristics were collected and analyzed: patient age, disease interval (time between diagnosis of cervical cancer and discovery of brain metastasis), clinical presentation, histopathology, location of brain lesions, treatment, and survival.
Statistical analysis
Wilcoxon Rank-Sum tests were performed on median data; Fischer’s exact tests were performed across frequency data. As is common practice, a significance threshold was set at P < 0.05. Survival was determined using the Kaplan–Meier analysis with a 95% confidence interval. Identifying information for patients alive at the time of publication of the respective articles was censored in the statistical analysis. All data were analyzed by custom scripts written in MATLAB.
RESULTS
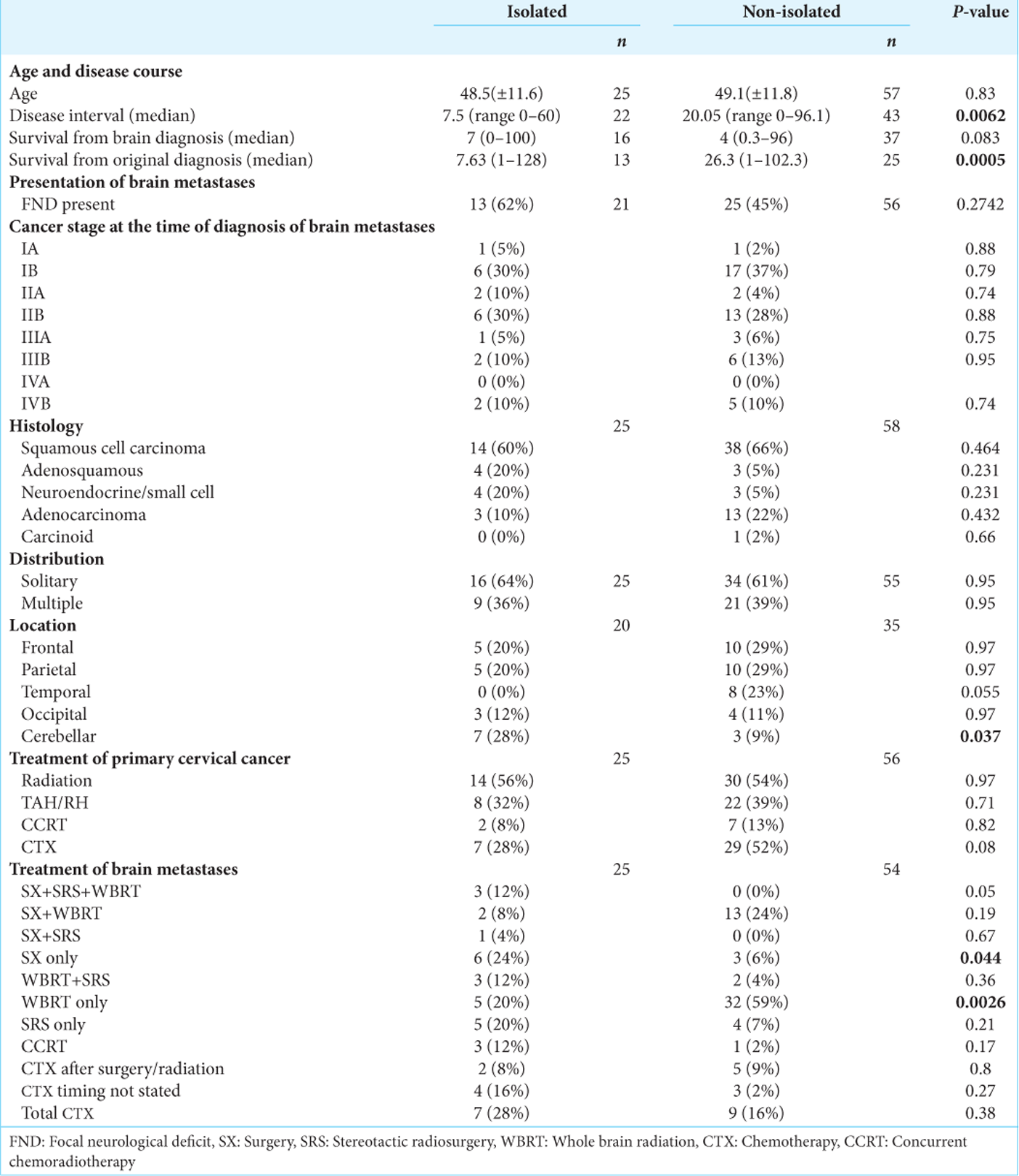
We identified 238 articles using the selected keywords, and 45 articles matched the topic of cerebral metastases from cervical cancer. Of these articles, 36 articles regarding cervical cancer with brain metastasis published between 1983 and 2018 met the study criteria and included granular patient data with information regarding survival. Twenty-five patients with IBM and 64 patients with NIBM were identified. The mean age in patients with IBM was 48.5 (range ± 11.6 years) and NIBM 49.1 (range ± 11.8 years) was not significantly different (P = 0.83). Cancer stage at the time of diagnosis of brain metastasis did not differ significantly between groups (mean stage IBM 2.1, NIBM 2.2, P = 0.71). The interval between cervical and brain lesion diagnosis was significantly shorter in the IBM group (7.5 months vs 20.05 months, IBM vs. NIBM, respectively; P = 0.006). Comparisons between IBM and NIBM are summarized in
Histopathology
Squamous cell carcinoma was the most common histopathology in both groups followed by adenosquamous and neuroendocrine tumors. The type of histology did not differ significantly between the two groups [
Treatment
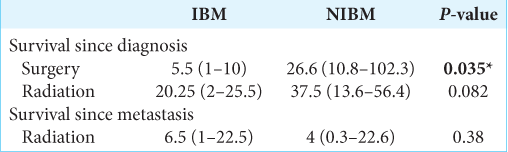
Radiation therapy was the most often used treatment for primary cervical cancer in both groups (56%, 14/25 IBM; 54%, 30/56 NIBM); the standard of care for radiotherapy in locally advanced cervical cancer is external-beam radiotherapy or cervical brachytherapy; institutional, patient, and provider preference largely determine modality usage. Surgical resection (total abdominal hysterectomy) was used in 32% (8/25) of patients with IBM and 39% (22/56) of patients with NIBM. About 48% (12/25) of patients with IBM and 30% (16/54) of patients with NIBM underwent surgical resection of brain metastases, while 52% (13/25) of IBM patients and 87% (47/54) of patients with NIBM underwent whole-brain radiation therapy [
Survival
We examined two separate time intervals: survival time from diagnosis of initial cervical cancer, and survival time from diagnosis of brain metastasis. The median overall survival from the time of initial diagnosis of cervical cancer was significantly shorter in IBM versus NIBM [IBM 7.63 months; NIBM, 26.3 months; P = 0.0005;
Temporal analysis showed that there is no significant effect from changing treatment modalities on patient outcomes over the years from which studies were collected [
DISCUSSION
Metastasis to the brain from cervical cancer is not common in clinical neurosurgical practice. As such, the possibility of brain metastasis is often not considered until there is evidence of neurological deficit. Based on data from the National Cancer Institute from 2009 to 2013, squamous cell carcinomas comprised 64% of all cervical cancers while adenocarcinomas comprised 15.1% and adenosquamous carcinomas comprised 3.4% of all reported cervical cancers.[
Many patients are at an advanced stage of the disease by the time brain lesions are diagnosed. Still, in this comparative analysis, 28.7% of patients with metastatic brain disease from primary cervical cancer were found to have no other distant metastases. Histopathology, patient age, symptomatology, and location of metastases were not significantly different between patients with isolated and NIBM.
At present, routine brain imaging is not a part of the guidelines for surveillance of post-treatment cervical cancer patients as issued by the American Society of Clinical Oncology or the National Comprehensive Cancer Network because of the very low incidence of brain metastases in gynecological cancer patients.[
The average age of patients with IBM in the present study was 48.5 years with no significant difference from the age in patients with NIBM (49.1 years). This distribution is consistent with previous reviews[
We found the overall median survival from diagnosis of brain metastasis across both groups was 4.6 months, similar to Teke et al.’s finding of 4.1 months.[
It is important to keep in mind, however, that as with all meta-analyses our current work may suffer from publication bias – that is to say, isolated brain metastases from cervical cancer are relatively rare and may, therefore, be seen as more reportable. However, we find the result that patients with IBM have reduced overall survival relative to NIBM patients despite the inherently greater disease burden of NIBM patients to be counterintuitive and interesting on its face. While outside the scope of the present study, one must wonder if IBM patients suffer from molecular and/or genetically distinct tumors than those with NIBM. We suggest two paths forward to answer this question: (1) a population-based prospective study to confirm or challenge the results of this meta-analysis, and (2) a molecular biological study of tumor samples from case-matched IBM and NIBM patients. In addition, the cumulative intracranial volume has been shown to be a prognostic factor for brain metastases from renal cell carcinoma.[
CONCLUSIONS
We have reviewed 25 cases of IBM from cervical cancer and have compared patient characteristics, treatment, and survival to data obtained from 64 cases of NIBM in cervical cancer. We found that the two groups have similar overall survival after brain metastasis, but as metastasis occur earlier in IBM, this group has reduced overall survival compared to NIBM in our pooled analysis. This runs somewhat counter to the notion that mortality is in part a function of overall tumor burden. It should aid neurosurgeons and other care providers in treatment planning and managing patient expectations.
References
1. Agrawal A, Kumar A, Sinha AK, Kumar M, Pandey SR, Khaniya S. Intracranial metastases from carcinoma of the cervix. Singapore Med J. 2007. 48: e154-6
2. Ali MA, Hirshman BR, Wilson B, Schupper AJ, Joshi R, Proudfoot JA. Improving the prognostic value of disease-specific graded prognostic assessment model for renal cell carcinoma by incorporation of cumulative intracranial tumor volume. World Neurosurg. 2017. 108: 151-6
3. Amita M, Sudeep G, Rekha W, Yogesh K, Hemant T. Brain metastasis from cervical carcinoma a case report. MedGenMed. 2005. 7: 26-
4. Branch BC, Henry J, Vecil GG. Brain metastases from cervical cancer a short review. Tumori. 2014. 100: e171-9
5. Brown Iii JV, Epstein HD, Kim R, Micha JP, Rettenmaier MA, Mattison JA. Rapid manifestation of CNS metastatic disease in a cervical carcinoma patient: A case report. Oncology. 2007. 73: 273-6
6. .editors. Cancer Statistics Review, 1975-2014 SEER Statistics. p.
7. Chung SB, Jo KI, Seol HJ, Nam DH, Lee JI. Radiosurgery to palliate symptoms in brain metastases from uterine cervix cancer. Acta Neurochir (Wien). 2013. 155: 399-405
8. Chura JC, Shukla K, Argenta PA. Brain metastasis from cervical carcinoma. Int J Gynecol Cancer. 2007. 17: 141-6
9. Cordeiro JG, Prevedello DM, da Silva Ditzel LF, Pereira CU, Araújo JC. Cerebral metastasis of cervical uterine cancer: Report of three cases. Arq Neuropsiquiatr. 2006. 64: 300-2
10. Cormio G, Colamaria A, Di Vagno G, De Tommasi A, Loverro G, Selvaggi L. Surgical decompression and radiation therapy in epidural metastasis from cervical cancer. Eur J Obstet Gynecol Reprod Biol. 2000. 89: 59-61
11. Divine LM, Kizer NT, Hagemann AR, Pittman ME, Chen L, Powell MA. Clinicopathologic characteristics and survival of patients with gynecologic malignancies metastatic to the brain. Gynecol Oncol. 2016. 142: 76-82
12. Elit L, Fyles AW, Oliver TK, Devries-Aboud MC, Fung-Kee-Fung M, members of the Gynecology Cancer Disease Site Group of Cancer Care Ontario’s Program in Evidence-Based Care. Follow-up for women after treatment for cervical cancer. Curr Oncol. 2010. 17: 65-9
13. Erdis E. A rare metastatic region of cervix cancer; the brain. J Pak Med Assoc. 2014. 64: 89-90
14. Fagundes H, Perez CA, Grigsby PW, Lockett MA. Distant metastases after irradiation alone in carcinoma of the uterine cervix. Int J Radiat Oncol Biol Phys. 1992. 24: 197-204
15. Fantini J, Sartori A, Manganotti P. Avoiding misdiagnosis: Cystic calcified brain metastases of uterine cervical cancer mimicking neurocysticercosis. BMJ Case Rep. 2017. 2017: bcr2016217952-
16. Forsyth PA, Posner JB. Headaches in patients with brain tumors: A study of 111 patients. Neurology. 1993. 43: 1678-83
17. Gaussmann AB, Imhoff D, Lambrecht E, Menzel C, Mose S. Spontaneous remission of metastases of cancer of the uterine cervix. Onkologie. 2006. 29: 159-61
18. Gressel GM, Lundsberg LS, Altwerger G, Katchi T, Azodi M, Schwartz PE. Factors predictive of improved survival in patients with brain metastases from gynecologic cancer: A single institution retrospective study of 47 cases and review of the literature. Int J Gynecol Cancer. 2015. 25: 1711-6
19. Gupta S, Bandzar S, Atallah H. Atypical presentation of cervical carcinoma with cerebral metastasis. Ochsner J. 2016. 16: 548-50
20. Huang CC, Kashima ML, Chen H, Shih IM, Kurman RJ, Wu TC. HPV in situ hybridization with catalyzed signal amplification and polymerase chain reaction in establishing cerebellar metastasis of a cervical carcinoma. Hum Pathol. 1999. 30: 587-91
21. Hwang JH, Yoo HJ, Lim MC, Seo SS, Kang S, Kim JY. Brain metastasis in patients with uterine cervical cancer. J Obstet Gynaecol Res. 2013. 39: 287-91
22. Ikeda SI, Yamada T, Katsumata N, Hida K, Tanemura K, Tsunematu R. Cerebral metastasis in patients with uterine cervical cancer. Jpn J Clin Oncol. 1998. 28: 27-9
23. Kasper E, Ippen F, Wong E, Uhlmann E, Floyd S, Mahadevan A. Stereotactic radiosurgery for brain metastasis from gynecological malignancies. Oncol Lett. 2017. 13: 1525-8
24. Kumar L, Tanwar RK, Singh SP. Intracranial metastases from carcinoma cervix and review of literature. Gynecol Oncol. 1992. 46: 391-2
25. Lefkowitz D, Asconapé J, Biller J. Intracranial metastases from carcinoma of the cervix. South Med J. 1983. 76: 519-21
26. Mahmoud-Ahmed AS, Suh JH, Barnett GH, Webster KD, Kennedy AW. Tumor distribution and survival in six patients with brain metastases from cervical carcinoma. Gynecol Oncol. 2001. 81: 196-200
27. Marongiu A, Salvati M, D’Elia A, Arcella A, Giangaspero F, Esposito V. Single brain metastases from cervical carcinoma: Report of two cases and critical review of the literature. Neurol Sci. 2012. 33: 937-40
28. Nagar YS, Shah N, Rawat S, Kataria T. Intracranial metastases from adenocarcinoma of cervix: A case report. Int J Gynecol Cancer. 2005. 15: 561-3
29. Nasu K, Satoh T, Nishio S, Nagai Y, Ito K, Otsuki T. Clinicopathologic features of brain metastases from gynecologic malignancies: A retrospective study of 139 cases (KCOG-G1001s trial). Gynecol Oncol. 2013. 128: 198-203
30. Omari-Alaoui HE, Gaye PM, Kebdani T, El Ghazi E, Benjaafar N, Mansouri A. Cerebellous metastases in patients with uterine cervical cancer. Two cases reports and review of the literature. Cancer Radiother. 2003. 7: 317-20
31. Park SH, Ro DY, Park BJ, Kim YW, Kim TE, Jung JK. Brain metastasis from uterine cervical cancer. J Obstet Gynaecol Res. 2010. 36: 701-4
32. Peters P, Bandi H, Efendy J, Perez-Smith A, Olson S. Rapid growth of cervical cancer metastasis in the brain. J Clin Neurosci. 2010. 17: 1211-2
33. Pyeon SY, Park JY, Ulak R, Seol HJ, Lee JM. Isolated brain metastasis from uterine cervical cancer: A case report and review of literature. Eur J Gynaecol Oncol. 2015. 36: 602-4
34. Rhiem K, Possover M, Gossmann A, Drebber K, Mallmann P, Ulrich U. “Occult” neuroendocrine component and rare metastatic pattern in cervical cancer: Report of a case and brief review of the literature. Eur J Gynaecol Oncol. 2007. 28: 139-41
35. Robinson JB, Morris M. Cervical carcinoma metastatic to the brain. Gynecol Oncol. 1997. 66: 324-6
36. Sato Y, Tanaka K, Kobayashi Y, Shibuya H, Nishigaya Y, Momomura M. Uterine cervical cancer with brain metastasis as the initial site of presentation. J Obstet Gynaecol Res. 2015. 41: 1145-8
37. Seidel R, Steinfeld A. Carcinoid of the cervix: Natural history and implications for therapy. Gynecol Oncol. 1988. 30: 114-9
38. Setoodeh R, Hakam A, Shan Y. Cerebral metastasis of cervical cancer, report of two cases and review of the literature. Int J Clin Exp Pathol. 2012. 5: 710-4
39. Tajran D, Berek JS. Surgical resection of solitary brain metastasis from cervical cancer. Int J Gynecol Cancer. 2003. 13: 368-70
40. Teke F, Tunc SY, Teke M, Turan Y, Urakci Z, Eren B. The impact of the stage and tumor size on rare brain metastasis of cervical cancer. Turk Neurosurg. 2016. 26: 818-23
41. Tenjarla S, Chawla S, Toy EP. Synchronous presentation of cavernous sinus metastasis and cervical cancer: A case report and review of literature. World J Oncol. 2014. 5: 228-31
42. Weltman E, Salvajoli JV, Brandt RA, de Morais Hanriot R, Prisco FE, Cruz JC. Radiosurgery for brain metastases: Who may not benefit?. Int J Radiat Oncol Biol Phys. 2001. 51: 1320-7
43. Ziainia T, Resnik E. Hemiballismus and brain metastases from squamous cell carcinoma of the cervix. Gynecol Oncol. 1999. 75: 289-92