- Division of Neurosurgery, Beth Israel Deaconess Medical Center and Harvard Medical School, Boston, Massachusetts, USA
- Department of Neurosurgery, National Neuroscience Institute, Riyadh, Saudi Arabia
- Department of Neurosurgery, Vanderbilt University Medical Center, Nashville, Tennessee, USA
- Department of Neurosurgery, Massachusetts General Hospital, Boston, Massachusetts, USA
- Department of Neurosurgery, University of Minnesota, Minneapolis, Minnesota, USA
Correspondence Address:
Ekkehard M. Kasper
Division of Neurosurgery, Beth Israel Deaconess Medical Center and Harvard Medical School, Boston, Massachusetts, USA
DOI:10.4103/sni.sni_400_17
Copyright: © 2018 Surgical Neurology International This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.How to cite this article: Mohamed M. Salem, Abdulrahman Y. Alturki, Matthew R. Fusco, Ajith J. Thomas, Bob S. Carter, Clark C. Chen, Ekkehard M. Kasper. Carotid artery stenting vs. carotid endarterectomy in the management of carotid artery stenosis: Lessons learned from randomized controlled trials. 16-Apr-2018;9:85
How to cite this URL: Mohamed M. Salem, Abdulrahman Y. Alturki, Matthew R. Fusco, Ajith J. Thomas, Bob S. Carter, Clark C. Chen, Ekkehard M. Kasper. Carotid artery stenting vs. carotid endarterectomy in the management of carotid artery stenosis: Lessons learned from randomized controlled trials. 16-Apr-2018;9:85. Available from: http://surgicalneurologyint.com/surgicalint-articles/carotid-artery-stenting-vs-carotid-endarterectomy-in-the-management-of-carotid-artery-stenosis-lessons-learned-from-randomized-controlled-trials/
Abstract
Background:Carotid artery stenosis, both symptomatic and asymptomatic, has been well studied with several multicenter randomized trials. The superiority of carotid endarterectomy (CEA) to medical therapy alone in both symptomatic and asymptomatic carotid artery stenosis has been well established in previous trials in the 1990s. The consequent era of endovascular carotid artery stenting (CAS) has offered another option for treating carotid artery stenosis. A series of randomized trials have now been conducted to compare CEA and CAS in the treatment of carotid artery disease. The large number of similar trials has created some confusion due to inconsistent results. Here, the authors review the trials that compare CEA and CAS in the management of carotid artery stenosis.
Methods:The PubMed database was searched systematically for randomized controlled trials published in English that compared CEA and CAS. Only human studies on adult patients were assessed. The references of identified articles were reviewed for additional manuscripts to be included if inclusion criteria were met. The following terms were used during search: carotid stenosis, endarterectomy, stenting. Retrospective or single-center studies were excluded from the review.
Results:Thirteen reports of seven large-scale prospective multicenter studies, comparing both interventions for symptomatic or asymptomatic extracranial carotid artery stenosis, were identified.
Conclusions:While the superiority of intervention to medical management for symptomatic patients has been well established in the literatures, careful selection of asymptomatic patients for intervention should be undertaken and only be pursued after institution of appropriate medical therapy until further reports on trials comparing medical therapy to intervention in this patient group are available.
Keywords: Asymptomatic, stroke, symptomatic, transient ischemic attack
INTRODUCTION AND BACKGROUND
Carotid artery stenosis, both symptomatic and asymptomatic, occupies a unique place among surgically treatable diseases. While the medical literature demonstrates many examples of large prospective multicenter randomized trials comparing various treatment regimens, the surgical literature has lagged somewhat behind in this regard. The reasons for this are many, and beyond the scope of this article. However, carotid artery stenosis is one surgically treatable disease that has been meticulously studied with robust prospective multicenter randomized trials. Trials such as the North American Symptomatic Carotid Endarterectomy Trial (NASCET),[
MATERIALS AND METHODS
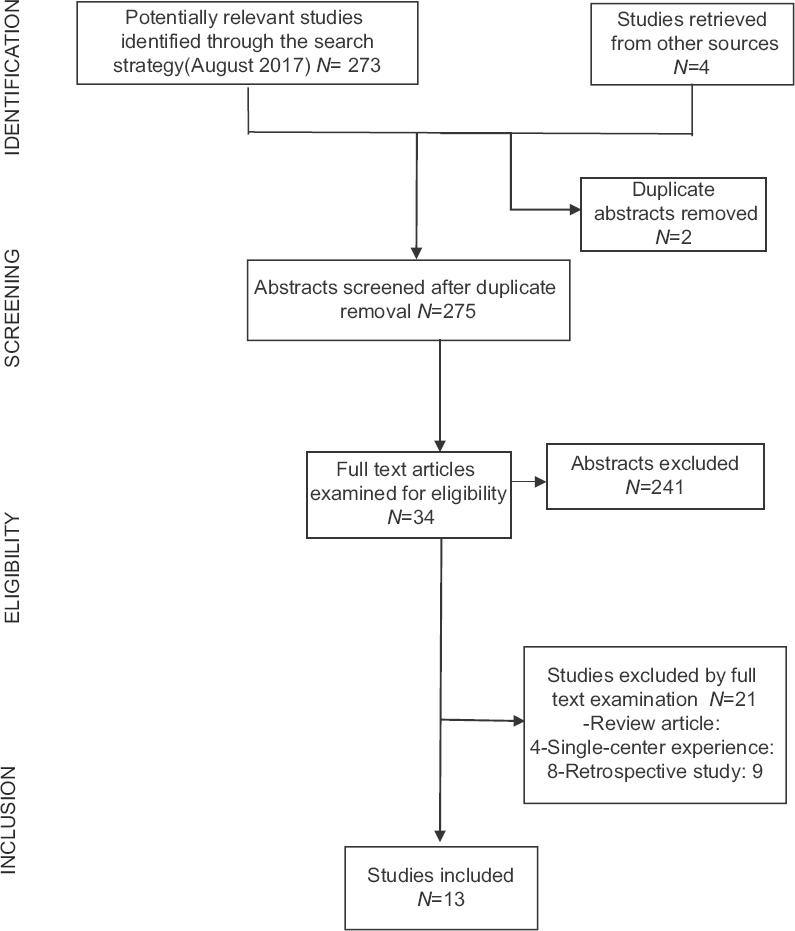
Our research question was defined using the patient, interventions, comparisons, and outcomes strategy. The aim of this review is to identify the current literature body of class I evidence, directly comparing CEA to CAS in patients with extracranial carotid stenosis in regards to peri-procedural complications and long-term outcomes. The PubMed database was searched for English articles through August 2017, using the following terms: “carotid stenosis,” “endarterectomy,” “stenting.” Only studies published after 1991 were considered, in order to cover the magnetic resonance imaging era. The references of retrieved articles were also reviewed to identify possible additional publications if inclusion criteria were met. The initial search yielded 275 articles of which 241 (87.6%) were excluded by abstract screening [
SUMMARY OF RANDOMIZED CLINICAL TRIALS
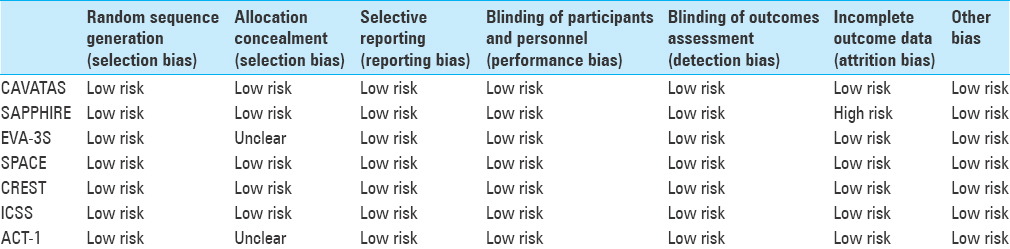
Endovascular vs. surgical treatment in patients with carotid stenosis in the carotid and vertebral artery transluminal angioplasty study (CAVATAS)
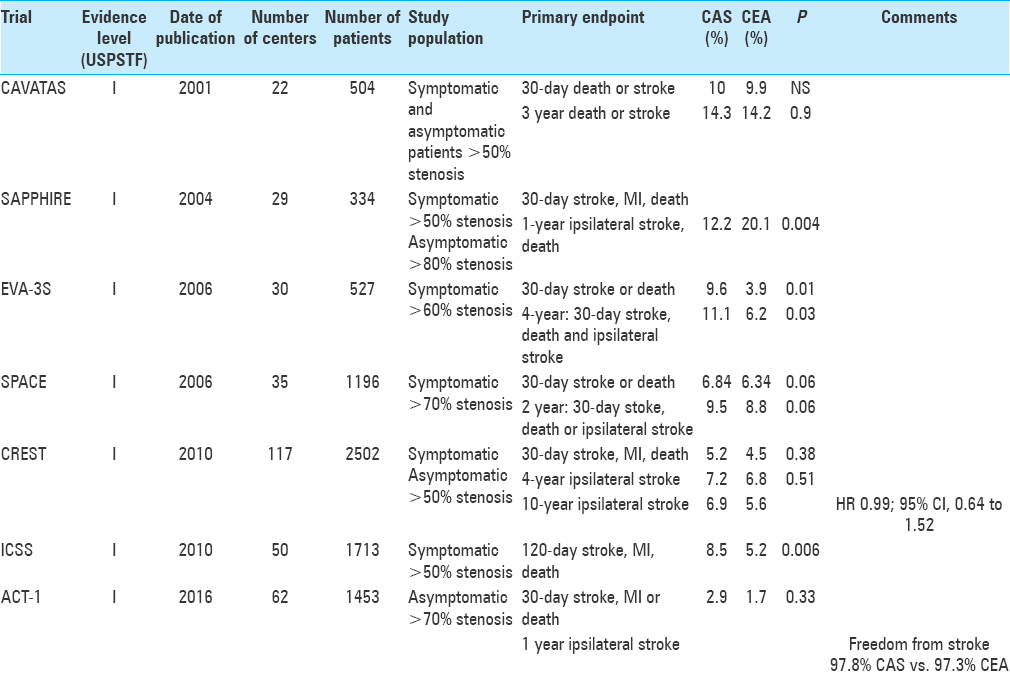
This was the first of these trials to report its results in 2001, randomly assigning 504 patients who were equally eligible for both surgery (n = 253) and stenting (n = 251). In the successfully treated endovascular arm, stent was used in 26% of cases, while balloon angioplasty alone was used in 74% of patients. The 30-day rate of stroke or death did not differ between the surgery and the stenting group (9.9% vs. 10%). The 1-year follow-up showed a higher ipsilateral restenosis rate in the endovascular arm (P < 0.0001). However, no differences in the ipsilateral stroke rate between the two groups were noted at the 3-year follow-up appointment [adjusted heart rate (HR) =1.04, 95% confidence interval (CI) 0.63–1.70, P = 0.9].[
Endarterectomy vs. angioplasty in patients with symptomatic severe carotid stenosis (EVA-3S)
This trial compared CAS and CEA in symptomatic patients with 60% stenosis or higher. The study was designed as a noninferiority study with a goal patient accrual of 872 patients. The primary endpoint was stroke or death within 30 days of treatment. The trial was stopped early after 527 patients were enrolled due to safety concerns as patients in the CAS group were demonstrated to suffer from higher rates of both stroke and death. The 30-day relative rate of stroke or death was 2.5 when CAS was compared to CEA (95% CI 1.2–5.1).[
Stenting and angioplasty with protection in patients at high risk for endarterectomy (SAPPHIRE)
The study evaluated the risk of peri-procedural events in high-risk CAS and CEA patients. Again, this trial was designed as a noninferiority study in both symptomatic patients with 50% or higher stenosis as well as asymptomatic patients with 80% or higher narrowing. The primary composite endpoints were defined as the rate of death, stroke, or myocardial infarction (MI) at 30 days as well as the 1-year rate of death or ipsilateral stroke. The absolute difference in primary endpoints was 7.9% lower with CAS (20 patients) compared to CEA (32 patients) (P = 0.004 for noninferiority). However, the results proved significant only in the asymptomatic group, while the results proved equivalent in symptomatic patients.[
Stent-supported percutaneous angioplasty of the carotid artery vs. endarterectomy (SPACE)
Also designed as a noninferiority trial, this study enrolled symptomatic patients with stenosis of 70% or higher within 180 days of either transient ischemic attack (TIA) or stroke. The primary endpoint of 30-day ipsilateral stroke or death was reached in 6.84% CAS patients and 6.34% CEA patients (P = 0.09), thus failing to result in significant evidence to demonstrate noninferiority of CAS to CEA.[
Carotid revascularization endarterectomy vs. stenting trial (CREST)
This remains the largest multicenter randomized trial directly comparing CAS and CEA to-date. Sponsored by the National Institute of Health (NIH), the trial enrolled 2502 patients at 108 centers in the United States and Canada. The most notable difference in the design of this trail was that any proceduralist participating in trial centers (whether surgeon or interventionalist), had to meet a rigorous set of standards to be allowed to participate in the trial. Both symptomatic and asymptomatic patients were enrolled. Here, symptomatic patients had to demonstrate a 50% or higher carotid stenosis on angiography or >70% stenosis on ultrasound, computed tomography angiography (CTA), or magnetic resonance angiography (MRA), while asymptomatic patients had to demonstrate a 60% or higher stenosis on angiography, 70% or higher on ultrasound, and 80% or higher on CTA or MRA. The primary composite endpoint was defined as the rate of stroke, MI, or death at 30 days or ipsilateral stroke within 4 years. There was no statistically significant difference demonstrated in the rate of primary endpoints between CAS (7.2%) and CEA (6.8%) (P = 0.51), neither in the symptomatic nor in the asymptomatic group. However, significant subgroup differences were seen in the rate of MI between CAS and CEA (1.1 and 2.3%, P = 0.03) and peri-procedural stroke (4.1 and 2.3%, P = 0.01). The increased rate of stroke was attributed to increased risk of events in elderly patients with CAS due to significant vessel tortuosity.[
Recently, the 10-year follow-up results of CREST were published.[
International carotid stenting study (ICSS)
This study evaluated the long-term efficacy of CAS vs. CEA in 1713 patients at 50 centers worldwide. Enrolled patients had to be symptomatic and demonstrate a 50% or greater stenosis and deemed well suited for either treatment. Again, endpoints of death or disabling strokes did not differ between the groups (HR 1.06, 95% CI 0.72–1.57, P = 0.77). However, the rates for any stroke (whether disabling or nondisabling) were more frequent with CAS when compared to CEA (HR 1.71, 95% CI 1.28–2.30, P < 0.001). Functional outcomes (as measured by Modified Rankin Score) at 1 year, 5 years, or final follow-up did not differ between the groups, indicating the increased stroke rate in CAS did not result in significantly disabling strokes.[
Asymptomatic carotid trial-1 (ACT-1)
Being the most recent of the retrieved studies, ACT-1 was originally designed to complement the CREST trial by comparing CEA to CAS in asymptomatic patients with carotid stenosis. The study was initially designed to enroll 1658 patients, but was stopped early after accrual of 1453 patients (87%) due to slow enrollment.[
EXPERT OPINION
“The bigger question is not whether to use CEA or CAS for symptomatic disease but rather whether any procedural therapy should be offered for the less severely stenotic subgroups (in the 60–80% range) of asymptomatic patients”—Bob S. Carter, MD, PhD, Harvard University and MGH, Boston MA.
In CEA and CAS, we have two efficacious tools for dealing with symptomatic carotid stenosis based on a multiplicity of trials demonstrating noninferiority between the two therapies. Because the cervical carotid artery is readily accessible, and the associated morbidity of CEA is typically low, open techniques have not experienced the same degree of erosion in procedural volume by endovascular techniques as has been seen in other neurovascular or systemic vascular diseases. Frankly, the bigger question is not whether to use CEA or CAS for symptomatic disease but rather whether any procedural therapy should be offered for the less severely stenotic subgroups (in the 60–80% range) of asymptomatic patients. Future studies will help us further refine our understanding of what degree of stenosis can still justify a procedural intervention for an asymptomatic patient, who may equally benefit from modern medical management.
“CEA vs CAS: Where do we go from here?” This is a title intro for the expert opinion segment, highlighting the expected future directions around this debate, or in other words how should we move forward in regards to the ongoing debate about CEA and CAS.
Several studies have been published to compare both treatments, including 13 RCTs.[
DISCUSSION
CEA has been well established as an effective modality for treatment of carotid stenosis. Two main techniques are currently used for this procedure: conventional and eversion endarterectomy. The former involves obtaining proximal control over the common carotid artery, then distal control around external carotid artery and internal carotid artery (ICA). Subsequent longitudinal arteriotomy of ICA is followed by either a patch angioplasty or primary vessel closure. The patch method is more commonly used and has been associated with better outcome in some studies.[
Intraoperative monitoring of brain function to detect the potential ischemia resulting from ICA cross-clamping is an essential part of the procedure. Bypassing the surgical clamp via shunting (routine or selective) is used as a preventive measure by some surgeons. However, these shunts have been reported to have their own side effects such as distal embolization and carotid dissection.[
On the contrary, the ongoing advancements in the endovascular field have bolstered CAS as a widely accepted alternative to open surgery,[
The randomized prospective trials comparing CAS and CEA have in large part demonstrated fairly consistent data over the past decade. EVA-3S demonstrated significantly worse outcomes with CAS over CEA. However, as this was one of the earliest of these trials comparing the two modalities, less sophisticated materials were available for these patients. This is evident in the relatively low usage of EPDs, something that is now commonly utilized by most operators. Excluding the CAVATAS trial, which was underpowered to detect equality, the other five trials presented either demonstrated noninferiority between CAS and CEA (SAPPHIRE, CREST, ICSS, ACT-1) or came very close (SPACE). Thus, these randomized controlled multicenter trials have, by and large, demonstrated CAS as a comparably safe alternative to CEA in the treatment of carotid artery stenosis.
On further analysis of these trials, certain subgroups have been evaluated to help in patient selection. The CREST trial demonstrated equivalent results overall, but with increased rates of MI with CEA and stroke with CAS. The increased rate of MI in CEA has been attributed to the increased myocardial stress of the open operation as well as possibly the need for alterations in patients’ antiplatelet medications at the time of the operation. The increased rate of peri-procedural stroke with CAS was related to the patient's age; surprisingly, the older patients (>70 years old) demonstrated increased stroke rates with the less-invasive CAS over CEA. This is believed to be related to the increased tortuosity of the access vasculature in older patients. Thus, while CAS and CEA demonstrate similar safety profiles overall, it is our opinion that CAS should be chosen in patients with significant cardiac risk factors at the time of procedure, while CEA should be chosen in patients over 70 years old.
While these previous trials have demonstrated similar safety profiles with CAS and CEA, the question of true efficacy remains elusive. Treatment in symptomatic disease with either CAS or CEA is indicated and has been demonstrated to be highly effective. However, current evaluations of the natural history of asymptomatic carotid stenosis continue to demonstrate lower risks of strokes or TIAs than previous reported. This is generally attributed to increased utilization and effectiveness of medical therapy, namely antiplatelet and lipid-lowering medications.[
DISCLOSURE
The authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. . Carotid Revascularization Using Endarterectomy or Stenting Systems (CaRESS) phase I clinical trial: 1-year results. J Vasc Surg. 2005. 42: 213-9
2. . Executive Committee for the Asymptomatic Carotid Atherosclerosis Study. JAMA. 1995. 273: 1421-8
3. . Endovascular versus surgical treatment in patients with carotid stenosis in the Carotid and Vertebral Artery Transluminal Angioplasty Study (CAVATAS): A randomised trial. Lancet (London, England). 2001. 357: 1729-37
4. . Randomised trial of endarterectomy for recently symptomatic carotid stenosis: final results of the MRC European Carotid Surgery Trial (ECST). Lancet (London, England). 1998. 351: 1379-87
5. Abbott AL, Paraskevas KI, Kakkos SK, Golledge J, Eckstein HH, Diaz-Sandoval LJ. Systematic Review of Guidelines for the Management of Asymptomatic and Symptomatic Carotid Stenosis. Stroke. 2015. 46: 3288-301
6. Ballotta E, Renon L, Da Giau G, Toniato A, Baracchini C, Abbruzzese E. A Prospective Randomized Study on Bilateral Carotid Endarterectomy: Patching Versus Eversion. Ann Surg. 2000. 232: 119-25
7. Barnett HJM, Taylor DW, Haynes RB, Sackett DL, Peerless SJ, Ferguson GG. Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis. N Engl J Med. 1991. 325: 445-53
8. Bonati LH, Dobson J, Featherstone RL, Ederle J, van der Worp HB, de Borst GJ. Long-term outcomes after stenting versus endarterectomy for treatment of symptomatic carotid stenosis: The International Carotid Stenting Study (ICSS) randomised trial. Lancet (London, England). 2015. 385: 529-38
9. Bonati LH, Ederle J, McCabe DJ, Dobson J, Featherstone RL, Gaines PA. Long-term risk of carotid restenosis in patients randomly assigned to endovascular treatment or endarterectomy in the Carotid and Vertebral Artery Transluminal Angioplasty Study (CAVATAS): Long-term follow-up of a randomised trial. Lancet Neurol. 2009. 8: 908-17
10. Bonati LH, Fraedrich G. Age modifies the relative risk of stenting versus endarterectomy for symptomatic carotid stenosis--a pooled analysis of EVA-3S, SPACE and ICSS. Eur J Vasc Endovasc Surg. 2011. 41: 153-8
11. Brooks WH, Jones MR, Gisler P, McClure RR, Coleman TC, Breathitt L. Carotid angioplasty with stenting versus endarterectomy: 10-year randomized trial in a community hospital. JACC Cardiovasc Intervent. 2014. 7: 163-8
12. Brooks WH, McClure RR, Jones MR, Coleman TC, Breathitt L. Carotid angioplasty and stenting versus carotid endarterectomy: Randomized trial in a community hospital. Am Coll Cardiol. 2001. 38: 1589-95
13. Brott TG, Hobson RW, Howard G, Roubin GS, Clark WM, Brooks W. Stenting versus endarterectomy for treatment of carotid-artery stenosis. N Engl J Med. 2010. 363: 11-23
14. Brott TG, Howard G, Roubin GS, Meschia JF, Mackey A, Brooks W. Long-Term Results of Stenting versus Endarterectomy for Carotid-Artery Stenosis. N Engl J Med. 2016. 374: 1021-31
15. Cao P, Giordano G, De Rango P, Zannetti S, Chiesa R, Coppi G. A randomized study on eversion versus standard carotid endarterectomy: Study design and preliminary results: the Everest Trial. J Vasc Surg. 1998. 27: 595-605
16. Chang J, Ahn JE, Landsman N, Rhee K, Chun L, Patel KK. Efficacy of contemporary medical management for asymptomatic carotid artery stenosis. Am Surg. 2013. 79: 987-91
17. Constantinou J, Jayia P, Hamilton G. Best evidence for medical therapy for carotid artery stenosis. J Vasc Surg. 2013. 58: 1129-39
18. Eckstein HH, Ringleb P, Allenberg JR, Berger J, Fraedrich G, Hacke W. Results of the Stent-Protected Angioplasty versus Carotid Endarterectomy (SPACE) study to treat symptomatic stenoses at 2 years: A multinational, prospective, randomised trial. Lancet Neurol. 2008. 7: 893-902
19. Ederle J, Dobson J, Featherstone RL, Bonati LH, van der Worp HB, de Borst GJ. Carotid artery stenting compared with endarterectomy in patients with symptomatic carotid stenosis (International Carotid Stenting Study): An interim analysis of a randomised controlled trial. Lancet (London, England). 2010. 375: 985-97
20. Forssell C, Kitzing P, Bergqvist D. Cranial nerve injuries after carotid artery surgery. A prospective study of 663 operations. Eur J Vasc Endovasc Surg. 1995. 10: 445-9
21. Gurm HS, Yadav JS, Fayad P, Katzen BT, Mishkel GJ, Bajwa TK. Long-term results of carotid stenting versus endarterectomy in high-risk patients. N Engl J Med. 2008. 358: 1572-9
22. Higgins JP, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ (Clinical research ed). 2011. 343: d5928-
23. Kastrup A, Groschel K, Krapf H, Brehm BR, Dichgans J, Schulz JB. Early outcome of carotid angioplasty and stenting with and without cerebral protection devices: A systematic review of the literature. Stroke. 2003. 34: 813-9
24. Katras T, Baltazar U, Rush DS, Sutterfield WC, Harvill LM, Stanton PE. Durability of eversion carotid endarterectomy: Comparison with primary closure and carotid patch angioplasty. J Vasc Surg. 2001. 34: 453-8
25. Kuliha M, Roubec M, Prochazka V, Jonszta T, Hrbac T, Havelka J. Randomized clinical trial comparing neurological outcomes after carotid endarterectomy or stenting. Br J Surg. 2015. 102: 194-201
26. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. Ann Intern Med. 2009. 151: W65-94
27. Mannheim D, Weller B, Vahadim E, Karmeli R. Carotid endarterectomy with a polyurethane patch versus primary closure: A prospective randomized study. J Vasc Surg. 2005. 41: 403-
28. Mas JL, Chatellier G, Beyssen B, Branchereau A, Moulin T, Becquemin JP. Endarterectomy versus stenting in patients with symptomatic severe carotid stenosis. N Engl J Med. 2006. 355: 1660-71
29. Mas JL, Trinquart L, Leys D, Albucher JF, Rousseau H, Viguier A. Endarterectomy Versus Angioplasty in Patients with Symptomatic Severe Carotid Stenosis (EVA-3S) trial: Results up to 4 years from a randomised, multicentre trial. Lancet Neurol. 2008. 7: 885-92
30. Meier P, Knapp G, Tamhane U, Chaturvedi S, Gurm HS. Short term and intermediate term comparison of endarterectomy versus stenting for carotid artery stenosis: Systematic review and meta-analysis of randomised controlled clinical trials. BMJ (Clinical research ed). 2010. 340: c467-
31. Naylor AR, Bolia A, Abbott RJ, Pye IF, Smith J, Lennard N. Randomized study of carotid angioplasty and stenting versus carotid endarterectomy: A stopped trial. J Vasc Surg. 1998. 28: 326-34
32. Radak D, Radevic B, Sternic N, Vucurevic G, Petrovic B, Ilijevski N. Single center experience on eversion versus standard carotid endarterectomy: A prospective non-randomized study. Cardiovasc Surg (London, England). 2000. 8: 422-8
33. Rerkasem K, Rothwell PM. Patch angioplasty versus primary closure for carotid endarterectomy. Cochrane Database Syst Rev. 2009. 4: Cd000160-
34. Ringleb PA, Allenberg J, Bruckmann H, Eckstein HH, Fraedrich G, Hartmann M. 30 day results from the SPACE trial of stent-protected angioplasty versus carotid endarterectomy in symptomatic patients: A randomised non-inferiority trial. Lancet (London, England). 2006. 368: 1239-47
35. Rosenfield K, Matsumura JS, Chaturvedi S, Riles T, Ansel GM, Metzger DC. Randomized Trial of Stent versus Surgery for Asymptomatic Carotid Stenosis. N Engl J Med. 2016. 374: 1011-20
36. Silver FL, Mackey A, Clark WM, Brooks W, Timaran CH, Chiu D. Safety of stenting and endarterectomy by symptomatic status in the Carotid Revascularization Endarterectomy Versus Stenting Trial (CREST). Stroke. 2011. 42: 675-80
37. Steinbauer MG, Pfister K, Greindl M, Schlachetzki F, Borisch I, Schuirer G. Alert for increased long-term follow-up after carotid artery stenting: Results of a prospective, randomized, single-center trial of carotid artery stenting vs carotid endarterectomy. J Vasc Surg. 2008. 48: 93-8
38. Wiesmann M, Schopf V, Jansen O, Bruckmann H. Stent-protected angioplasty versus carotid endarterectomy in patients with carotid artery stenosis: Meta-analysis of randomized trial data. Eur Radiol. 2008. 18: 2956-66
39. Yadav JS, Wholey MH, Kuntz RE, Fayad P, Katzen BT, Mishkel GJ. Protected carotid-artery stenting versus endarterectomy in high-risk patients. N Engl J Med. 2004. 351: 1493-501
40. Zahn R, Mark B, Niedermaier N, Zeymer U, Limbourg P, Ischinger T. Embolic protection devices for carotid artery stenting: Better results than stenting without protection?. Eur Heart J. 2004. 25: 1550-8
41. Zhang L, Zhao Z, Ouyang Y, Bao J, Lu Q, Feng R. Systematic Review and Meta-Analysis of Carotid Artery Stenting Versus Endarterectomy for Carotid Stenosis: A Chronological and Worldwide Study. Medicine. 2015. 94: e1060-