- Department of Neurosurgery, KAT General Hospital of Attica, Kifisia 145 61, Greece
- Department of Pathology, KAT General Hospital of Attica, Kifisia 145 61, Greece
Correspondence Address:
Sotirios Apostolakis
Department of Neurosurgery, KAT General Hospital of Attica, Kifisia 145 61, Greece
DOI:10.4103/sni.sni_212_18
Copyright: © 2018 Surgical Neurology International This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.How to cite this article: Sotirios Apostolakis, Athanasios Mitropoulos, Kalliopi Diamantopoulou, Konstantinos Vlachos. Cavernoma of the cauda equina. 28-Aug-2018;9:174
How to cite this URL: Sotirios Apostolakis, Athanasios Mitropoulos, Kalliopi Diamantopoulou, Konstantinos Vlachos. Cavernoma of the cauda equina. 28-Aug-2018;9:174. Available from: http://surgicalneurologyint.com/surgicalint-articles/cavernoma-of-the-cauda-equina/
Abstract
Background:Cavernomas are benign malformations of the vasculature. In the central nervous system, they are mostly located supratentorially. However, in adults, cavernomas also comprise about 3% of all subdural spinal cord tumors. Notably, cavernomas of the cauda equina are extremely rare, with only 23 cases reported in the literature. Here, we report the 24th case involving a 77-year-old male.
Case Description:A 77-year-old male presented with low back pain for 3 years duration. His history included prostate cancer, skin melanoma, and a sick sinus syndrome requiring a pacemaker. An enhanced computed tomography of the lumbar spine showed an inhomogeneously enhanced, intramedullary mass, located at the L3 level. The patient underwent an L3 hemilaminectomy with gross total excision of the lesion. Macroscopically, the tumor was mulberry-shaped and well demarcated. However, it was strongly adherent to a nerve root of the cauda equina which required resection. The histologic examination was consistent with a cavernoma. The patient subsequently fully recovered without a focal neurological deficit.
Conclusions:Cavernomas of the cauda equina are extramedullary, arise on the inner aspect of the dura, and may be tightly adhered to the nerve roots. To attain gross total excision, the involved nerve may have to be sacrificed; in some cases, this may result in a permanent neurological deficit. Of interest, half of the cauda equina lesions were previously found in patients who had prior radiotherapy; this was not the case in this patient.
Keywords: Cauda equina, cavernous hemangioma, radiation therapy
INTRODUCTION
Cavernous hemangiomas or cavernomas are benign malformations of the vasculature that can occasionally be found in the central nervous system (CNS). The vast majority of CNS cavernous hemangiomas are located supratentorially (69%), primarily in males (58.9%) and in patients in their 40s.
Spinal cavernomas are reported in only 3% of CNS cases. They are typically subdural in their location and are predominantly found in the thoracolumbar spine.[
CASE DESCRIPTION
A 77-year-old male patient presented with low back pain alone for 3 years duration. He had a history of prostate cancer, skin melanoma, and an MRI-incompatible DDI pacemaker implanted for sick sinus syndrome.
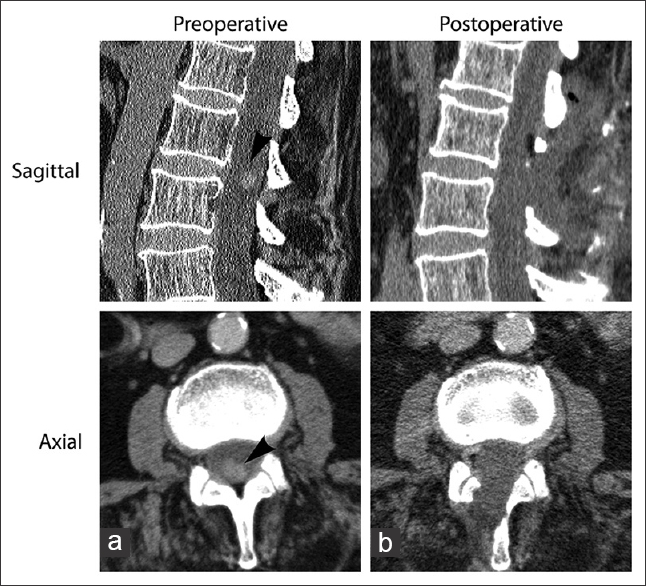
The enhanced lumbar computed tomography (CT) showed an inhomogeneously enhancing intramedullary mass, located at the L3 level, measuring approximately 1.3 × 1 × 1 cm [
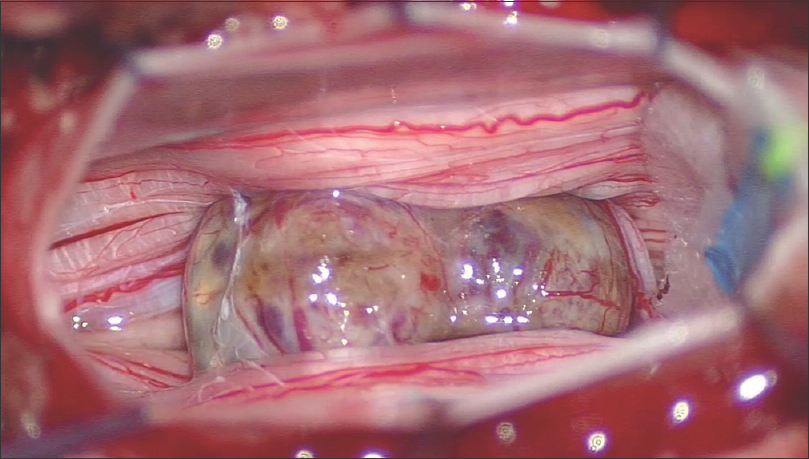
Surgical resection required an L3 hemilaminectomy; the lesion was fully excised. Macroscopically, the tumor was mulberry-shaped, purplish in color, and well demarcated, but tightly adhered to a nerve root of the cauda equina [
Figure 3
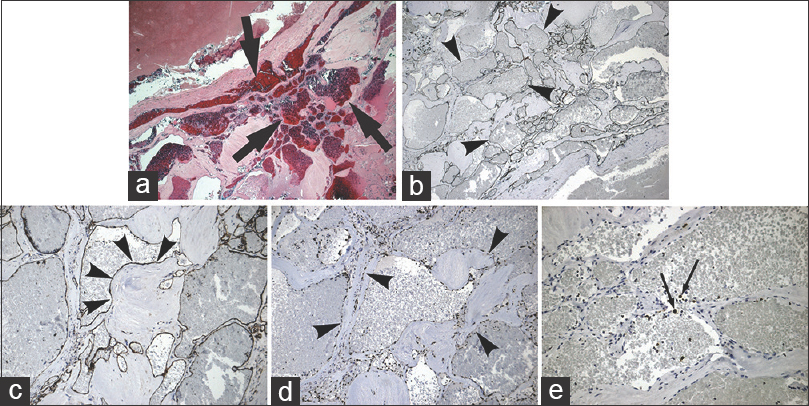
Histologic examination of the excised tissue revealed the presence of dilated cavernous vessels (image a, H and E, ×4) lined by endothelial cells without indications of atypia (image b, CD34 immunohistochemistry, magnification ×4; image c, CD31 immunohistochemistry, magnification ×10; image d, ERG immunohistochemistry, magnification ×10). Proliferation of cells as demonstrated by Ki-67 was scarce (image e, magnification ×10)
Immunohistochemical examination of tumor mass
Immunohistochemical examination of the specimen demonstrated that the endothelial lining was composed of a rich network of cavernous vessels (CD34 –
Postoperative course
Postoperatively, the patient's immediate and 6-month postoperative CT scans documented complete removal of the tumor, and he remained neurologically intact [
DISCUSSION
Cavernous malformations of the cauda equina are extremely rare and have been reported in only 23 cases.[
Anatomical and pathological findings
Cavernomas are mulberry-shaped, well-defined, unencapsulated lesions. They are composed of a rich network of dilated sinusoidal spaces lined by a single layer of endothelium which anastomose with each other.[
Cauda equina cavernomas
Cauda equina cavernomas are intradural, extramedullary lesions that may be tightly adherent to nerve roots which in some cases may have to be sacrificed to attain a gross total resection.[
Of interest, Drazin et al.[
CONCLUSIONS
Here, we report a cauda equina cavernoma in a 77-year-old male who presented with 3 years of low back pain alone. With a history of prostate cancer and skin melanoma, an enhanced CT of the lumbar spine was ordered. The lesion proved to be intradural, extramedullary cauda equina cavernoma which was densely adherent to a nerve root that had to be sacrificed to attain a gross total excision. Postoperatively, the immediate and 6-month enhanced CT studies confirmed no tumor recurrence, and the patient remained neurologically intact.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Chun SW, Kim SJ, Lee TH, Koo HS. Intra-root cavernous angioma of the Cauda equina: A case report and review of the literature. J Korean Neurosurg Soc. 2010. 47: 291-4
2. Drazin D, Kappel A, Withrow S, Perry T, Chu R, Phuphanich S. Post-irradiation lumbosacral radiculopathy associated with multiple cavernous malformations of the Cauda equina: Case report and review of the literature. Surg Neurol Int. 2017. 8: 26-
3. Imagama S, Ito Z, Ando K, Kobayashi K, Hida T, Ito K. Optimal timing of surgery for intramedullary cavernous hemangioma of the spinal cord in relation to preoperative motor paresis, disease duration, and tumor volume and location. Global Spine J. 2017. 7: 246-53
4. Lanfranconi S, Ronchi D, Ahmed N, Civelli V, Basilico P, Bresolin N. A novel CCM1 mutation associated with multiple cerebral and vertebral cavernous malformations. BMC Neurol. 2014. 14: 158-
5. Nowak DA, Widenka DC. Spinal intradural capillary haemangioma: A review. Eur Spine J. 2001. 10: 464-72
6. Petersen TA, Morrison LA, Schrader RM, Hart BL. Familial versus sporadic cavernous malformations: Differences in developmental venous anomaly association and lesion phenotype. AJNR Am J Neuroradiol. 2010. 31: 377-82
7. Wein S, Gaillard F. Intradural spinal tumours and their mimics: A review of radiographic features. Postgrad Med J. 2013. 89: 457-69
8. Winer LH. Hemangiomas; histologic structure, and treatment. Calif Med. 1952. 77: 242-7