- Department of Neurosurgery, Indiana University, Indianapolis, Indiana, USA
- Division of Neuropathology, Department of Pathology and Laboratory Medicine, Indiana University School of Medicine, Indianapolis, Indiana, USA
Correspondence Address:
Mahua Dey
Department of Neurosurgery, Indiana University, Indianapolis, Indiana, USA
DOI:10.4103/sni.sni_111_17
Copyright: © 2017 Surgical Neurology International This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Kaleigh Fetcko, Dibson D. Gondim, Jose M. Bonnin, Mahua Dey. Cervical cancer metastasis to the brain: A case report and review of literature. 09-Aug-2017;8:181
How to cite this URL: Kaleigh Fetcko, Dibson D. Gondim, Jose M. Bonnin, Mahua Dey. Cervical cancer metastasis to the brain: A case report and review of literature. 09-Aug-2017;8:181. Available from: http://surgicalneurologyint.com/surgicalint-articles/cervical-cancer-metastasis-to-the-brain-a-case-report-and-review-of-literature/
Abstract
Background:Intracranial metastasis from cervical cancer is a rare occurrence.
Methods:In this study we describe a case of cervical cancer metastasis to the brain and perform an extensive review of literature from 1956 to 2016, to characterize clearly the clinical presentation, treatment options, molecular markers, targeted therapies, and survival of patients with this condition.
Results:An elderly woman with history of cervical cancer in remission, presented 2 years later with a right temporo-parietal tumor, which was treated with surgery and subsequent stereotactic radiosurgery (SRS) to the resection cavity. She then returned 5 months later with a second solitary right lesion; she again underwent surgery and SRS to the resection cavity with no signs of recurrence 6 months later. According to the reviewed literature, the most common clinical presentation included females with median age of 48 years; presenting symptoms such as headache, weakness/hemiplegia/hemiparesis, seizure, and altered mental status (AMS)/confusion; multiple lesions mostly supratentorially located; poorly differentiated squamous cell carcinoma; and additional recurrences at other sites. The best approach to treatment is a multimodal plan, consisting of SRS or whole brain radiation therapy (WBRT) for solitary brain metastases followed by chemotherapy for systemic disease, surgery and WBRT for solitary brain lesions without systemic disease, and SRS or WBRT followed by chemotherapy for palliative care. The overall prognosis is poor with a mean and median survival time from diagnosis of brain metastasis of 7 and 4.6 months, respectively.
Conclusion:Future efforts through large prospective randomized trials are warranted to better describe the clinical presentation and identify more effective treatment plans.
Keywords: Brain metastasis, cervical cancer, intracranial metastasis
INTRODUCTION
Cervical cancer is one of the most malignant cancers affecting women, second only to breast cancer.[
Brain metastasis from cervical cancer is a rare occurrence. With only approximately 100 cases of reported intracranial metastases of cervical cancer in the literature, proper management of these patients remains unclear.[
CASE REPORT
A 75-year-old female with a history of stage IIIB squamous cell cancer of the cervix, which had been treated and in remission for about 2 years, presented in February 2016 with several weeks of decreased coordination and decreased balance with weakness and clumsiness noted especially on her left side in addition to a left facial droop. Magnetic resonance imaging (MRI) of her brain showed a solitary 4.6 cm × 3.4 cm × 4.1 cm heterogeneous solid mass at the right temporo-parietal junction with surrounding edema, mass effect, and early uncal herniation suggestive of either a metastasis or high-grade primary lesion [Figure
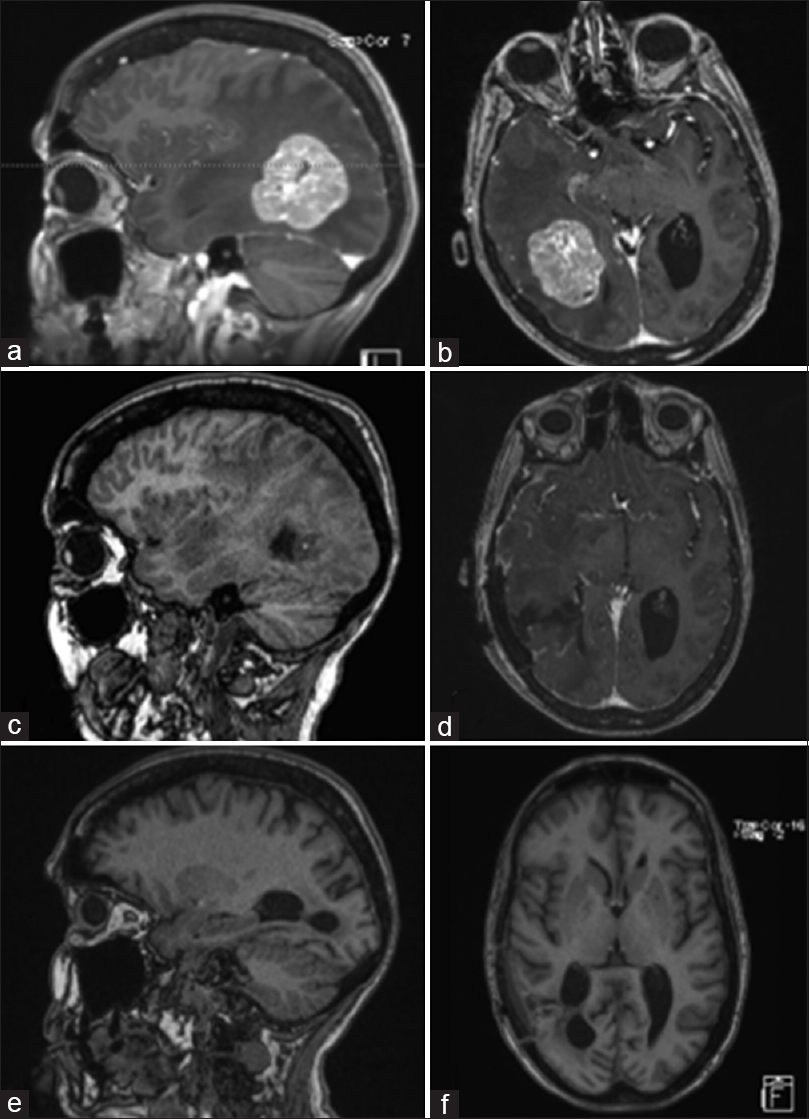
Figure 1
Pre-operative MRI of brain showing a solitary heterogeneously enhancing solid mass at the right temporal-parietal junction with surrounding edema, mass effect, and early uncal herniation (a and b). Immediate post-operative MRI of brain showing post-operative changes in right temporal-parietal area with gross total resection of the lesion (c and d). MRI of brain seven weeks after surgical resection showing no evidence of tumor progression, significantly improved edema around the resection area, and partially entrapped right occipital horn likely from intraventricular adhesive disease (e and f)
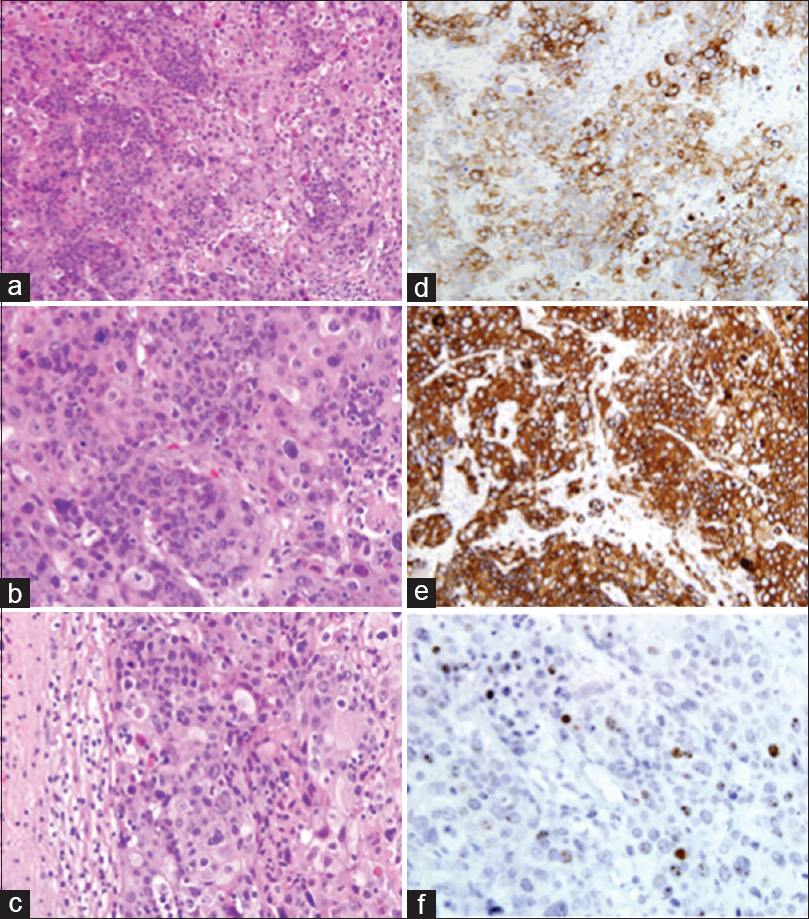
Figure 2
Squamous cell carcinoma of the uterine cervix, metastatic to the brain: marked anaplasia and extensive keratinization of tumor cells. H and E ×200 (a) and ×400 (b). Note the sharp demarcation between tumor tissue and the surrounding compressed cerebral parenchyma. H and E, ×400 (c). Immunohistochemical stains. Tumor cells are strongly positive for CK7 and CK5/6, ×400 (d and e). In-situ hybridization for HPV (f)
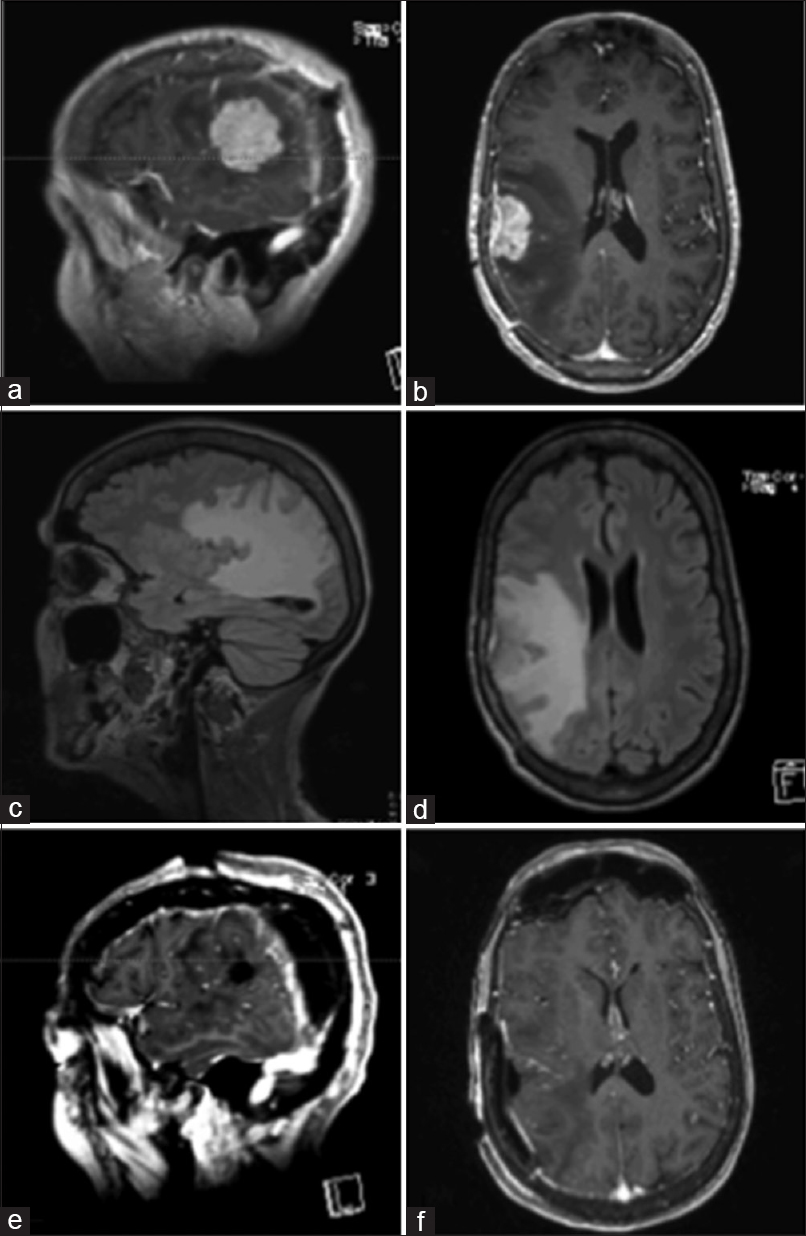
Postoperative MRI [Figure
In July 2016, the patient had a left-sided focal clonic seizure and an episode of left-sided weakness. An MRI showed a new single metastatic tumor measuring 2.3 × 3.5 cm2 noted in the right temporo-parietal area with significant surrounding edema within temporal lobe and extending into right parietal and occipital lobes [Figure
DISCUSSION
The incidence of cervical cancer metastasis to the brain has been reported as ranging from 0.4% to 2.3%.[
Clinical presentation
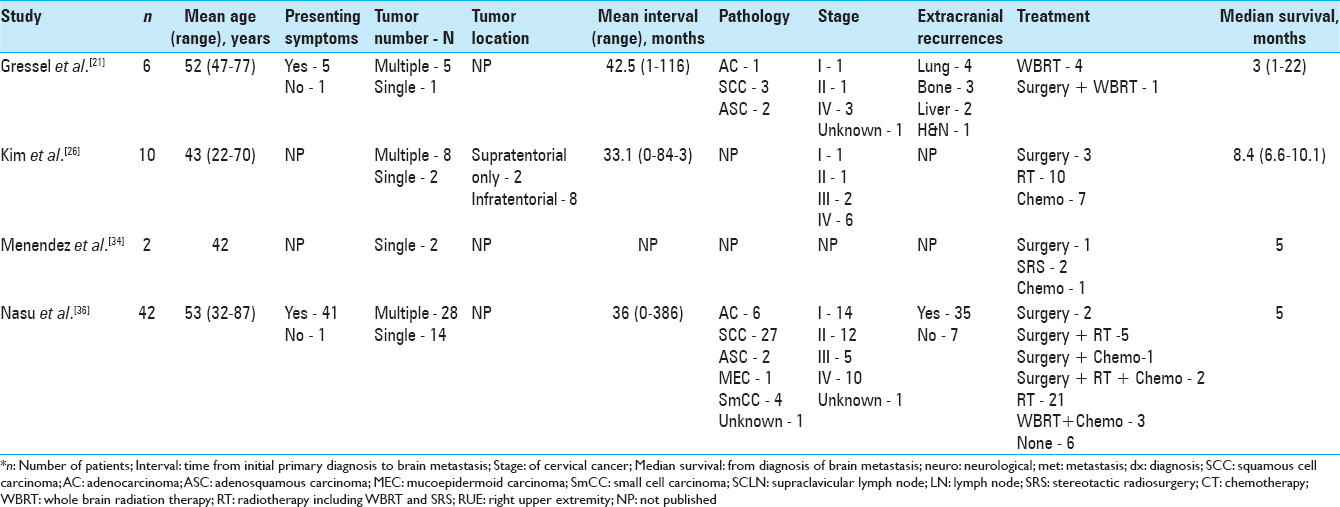
The median age of all the patients found in our literature review was 48 years, ranging from 29 to 87 years. Of the interval times and mean interval times reported by the articles from our literature review, the median interval time was 17.2 months. The interval time varied greatly with some patients diagnosed with brain metastasis at the time of their primary cancer diagnosis, while some experienced much longer intervals even up to 8 years. The patient from our case was a 75-year-old female with a 2-year interval time from primary diagnosis to brain metastasis diagnosis.
Of the reported symptoms of the patients from our literature review, the most frequent presenting symptoms included headache (31%), hemiparesis/hemiplegia/weakness (16%), seizure (11%), and altered mental status/confusion (9%). Slightly more than half of these patients (55%) experienced multiple lesions, while slightly less than half (45%) were found to have solitary lesions. Most of the brain metastases were supratentorial (75%) and were found in all the different lobes, and although less frequent, the most common area of infratentorial lesions was in the cerebellum. In our case, the patient presented in February 2016 with left-sided ataxia, weakness, facial droop, and an episode of confusion; she was found to have a solitary lesion located supratentorially in right temporo-parietal lobe. She then presented again in July 2016 after a left-sided focal clonic seizure and an episode of left-sided weakness with findings of another single metastatic lesion in right temporo-parietal lobe.
Mahmoud-Ahmed et al. noted that most brain metastases from cervical cancer are poorly differentiated and of various histologic types.[
Positive immunohistochemistry for CK7 is frequently seen with squamous cell carcinoma of the cervix, which the patient from our case report was found to have from initial brain lesion.[
Treatment
Similar to intracranial metastasis from other cancers, treatment of intracranial metastasis of cervical carcinoma includes surgery, radiation therapy, SRS, chemotherapy, or a combination of these therapies. Several of the patients from our literature review underwent surgical resection (35%), and many of them received whole brain radiation therapy (WBRT; 48%). However, there were many combinations of different therapies for the treatment plans of these patients, highlighting the lack of standard treatment protocol for this disease process. The most common treatment courses consisted of WBRT alone (17%) and surgical excision plus WBRT (13%); however, the best course of treatment is still not clear at this time with several studies showing benefits of certain multimodal treatment plans. Our literature review shows majority of the younger patients were treated with surgical resection; however, surgical resection in patients greater than 70 years is a rare occurrence. In our case, the patient was treated with surgery, followed by SRS to the resection cavity for both the metastatic lesions. No additional recurrences or new neurological symptoms were noted 6 months following her second tumor resection. We chose to treat with surgical resection in combination with SRS and avoided WBRT because of patients’ excellent performance status.
Surgical resection of cervical cancer metastasis to the brain is typically performed in patients with a solitary tumor or multiple adjacent tumors, patients with critically located or life-threatening metastases, or patients with diagnostic uncertainty.[
Chura et al. examined 12 cases of patients with intracranial metastases from cervical cancer treated with steroids, WBRT, surgery, or a combination of those therapies. The median survival from diagnosis of brain metastasis was 2.3 months (0.3–7.9 months); improved survival was observed in patients who had surgery and patients who underwent SRS with a median survival of 6.2 months vs. 1.3 months for patients treated with only WBRT (P = 0.024). Furthermore, chemotherapy seemed to improve survival with a median of 4.4 months in patients who received chemotherapy after WBRT compared to 0.9 months for patients who did not receive additional treatment after WBRT (P = 0.016).[
SRS appears to offer effective local tumor control for gynecologic malignancies with a study by Matsunaga et al.[
Chemotherapy plays a significant role in the treatment of cervical cancer, specifically cisplatin; however, its effects on the outcome of intracranial cervical cancer metastases is still not clear but may be used initially in the setting of multiple lesions.[
Prognosis
Although reported incidence of intracranial metastases from cervical cancer is low, autopsy reports have noted that up to 3–10% of cervical cancer patients have brain metastases, which brings to question if and when central nervous system screening should be performed.[
In the early stages of cervical cancer (stage I–IIb), there is a 5-year survival of 65–80% of patients, while there is a 0% 5-year survival with disseminated metastases.[
The outcome of patients with intracranial metastases from cervical cancer is influenced by the patient's neurological condition, length of clinical history, age, pathological subtype, number of tumors, and comorbidities; good prognostic factors include age <50 years, single brain metastasis, good performance status, and no extracranial metastases.[
New research is focusing on identifying molecular characteristics of gynecologic tumors in hopes of improving diagnosis, determining prognosis, and guiding treatment according to potentially targetable biomarkers.[
Additionally, signaling activation of the protein kinase mTOR, which is involved in protein synthesis, has been noted in both HPV-negative and HPV-positive cervical cancer tissues and cell lines; mTOR inhibitors have also shown to effectively decrease the activity of mTOR along with remarkably decreasing tumor burden.[
CONCLUSIONS
Cervical cancer metastasis to the brain is an infrequent event. According to our literature review, the median age of diagnosis for these patients was 48 years (29–87 years). The median time interval from primary diagnosis to diagnosis of intracranial metastases was 17.2 months with a wide range spanning from simultaneous diagnosis with primary cervical cancer diagnosis up to 8 years after primary cancer diagnosis. The most common presenting symptoms include headache, weakness/hemiplegia/hemiparesis, seizure, and altered mental status/confusion. The majority of patients were found to have multiple lesions that were mostly supratentorially located. The patients most commonly had poorly differentiated squamous cell carcinoma with additional recurrences at other sites—mainly the chest/lungs, bone, and abdomen/pelvis.
There is no standard treatment for this condition, and a various treatment options and combination of treatment options have been utilized such as surgical excision, WBRT, chemotherapy, and SRS. WBRT with or without surgery has been the most frequently used management. However, treatment should be individualized with the goal of providing symptomatic relief and improving quality of life. Aggressive treatment options should be based on patient's performance status and not age alone. A multimodal treatment plan is highly recommended as the best approach, specifically suggesting the use of SRS or WBRT for solitary brain metastases followed by chemotherapy for systemic disease, the use of surgical resection with WBRT for solitary brain lesions without systemic disease, and the use of SRS or WBRT and steroids followed by chemotherapy for palliative symptomatic relief.[
In general, intracranial cervical cancer metastasis carries poor prognosis. Favorable prognostic factors for patients with cervical cancer brain metastases include age <50 years, single brain metastasis, good performance status, and no extracranial metastases.[
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. . Integrated genomic and molecular characterization of cervical cancer. Nature. 2017. 543: 378-84
2. Agrawal A, Kumar A, Sinha AK, Kumar M, Pandey SR, Khaniya S. Intracranial metastases from carcinoma of the cervix. Singapore Med J. 2007. 48: e154-6
3. Amita M, Sudeep G, Rekha W, Yogesh K, Hemant T. Brain metastasis from cervical carcinoma—a case report. Med Gen Med. 2005. 7: 26-
4. Asimomytis A, Karanikou M, Rodolakis A, Vaiopoulou A, Tsetsa P, Creatsas G. mTOR downstream effectors, 4EBP1 and eIF4E, are overexpressed and associated with HPV status in precancerous lesions and carcinomas of the uterine cervix. Oncology Lett. 2016. 12: 3234-40
5. Azimirad A, Sarraf Z. Parkinsonism in a recurrent cervical cancer patient: Case report and review of the literature. J Fam Reprod Health. 2013. 7: 189-91
6. Branch BC, Henry J, Vecil GG. Brain metastases from cervical cancer – A short review. Tumori. 2014. 100: e171-9
7. Brown Iii JV, Epstein HD, Kim R, Micha JP, Rettenmaier MA, Mattison JA. Rapid manifestation of CNS metastatic disease in a cervical carcinoma patient: A case report. Oncology. 2007. 73: 273-6
8. Buchsbaum HJ, Rice AC. Cerebral metastasis in cervical carcinoma. Am J Obstet Gynecol. 1972. 114: 276-8
9. Chung SB, Jo KI, Seol HJ, Nam DH, Lee JI. Radiosurgery to palliate symptoms in brain metastases from uterine cervix cancer. Acta Neurochir. 2013. 155: 399-405
10. Chura JC, Shukla K, Argenta PA. Brain metastasis from cervical carcinoma. Int J Gynecol Cancer. 2007. 17: 141-6
11. Cordeiro JG, Prevedello DM, da Silva Ditzel LF, Pereira CU, Araujo JC. Cerebral metastasis of cervical uterine cancer: Report of three cases. Arq Neuropsiquiatr. 2006. 64: 300-2
12. Cormio G, Colamaria A, Loverro G, Pierangeli E, Di Vagno G, De Tommasi A. Surgical resection of a cerebral metastasis from cervical cancer: Case report and review of the literature. Tumori. 1999. 85: 65-7
13. Ding DC, Chu TY. Brain and intramedullary spinal cord metastasis from squamous cell cervical carcinoma. Taiwan J Obstet Gynecol. 2010. 49: 525-7
14. Divine LM, Kizer NT, Hagemann AR, Pittman ME, Chen L, Powell MA. Clinicopathologic characteristics and survival of patients with gynecologic malignancies metastatic to the brain. Gynecol Oncol. 2016. 142: 76-82
15. Erdis E. A rare metastatic region of cervix cancer; the brain. J Pak Med Assoc. 2014. 64: 89-90
16. Franco EL, Schlecht NF, Saslow D. The epidemiology of cervical cancer. Cancer J (Sudbury, Mass). 2003. 9: 348-59
17. Gao G, Smith DI. Human papillomavirus and the development of different cancers. Cytogenet Genome Res. 2016. 150: 185-93
18. Gaussmann AB, Imhoff D, Lambrecht E, Menzel C, Mose S. Spontaneous remission of metastases of cancer of the uterine cervix. Onkologie. 2006. 29: 159-61
19. Gaze MN, Gregor A, Whittle IR, Sellar RJ. Calcified cerebral metastasis from cervical carcinoma. Neuroradiology. 1989. 31: 291-
20. Gill TJ, Dammin GJ. A case of epidermoid carcinoma of the cervix uteri with cerebral metastasis. J Pathol Bacteriol. 1959. 78: 569-71
21. Gressel GM, Lundsberg LS, Altwerger G, Katchi T, Azodi M, Schwartz PE. Factors predictive of improved survival in patients with brain metastases from gynecologic cancer: A single institution retrospective study of 47 cases and review of the literature. Int J Gynecol Cancer. 2015. 25: 1711-6
22. Gupta S, Bandzar S, Atallah H. Atypical presentation of cervical carcinoma with cerebral metastasis. Ochsner J. 2016. 16: 548-50
23. Hwang JH, Yoo HJ, Lim MC, Seo SS, Kang S, Kim JY. Brain metastasis in patients with uterine cervical cancer. J Obstet Gynaecol Res. 2013. 39: 287-91
24. Ikeda S, Yamada T, Katsumata N, Hida K, Tanemura K, Tsunematu R. Cerebral metastasis in patients with uterine cervical cancer. Jap J Clin Oncol. 1998. 28: 27-9
25. Kim K, Cho SY, Kim BJ, Kim MH, Choi SC, Ryu SY. The type of metastasis is a prognostic factor in disseminated cervical cancer. J Gynecol Oncol. 2010. 21: 186-90
26. Kim YZ, Kwon JH, Lim S. A clinical analysis of brain metastasis in gynecologic cancer: A retrospective multi-institute analysis. J Korean Med Sci. 2015. 30: 66-73
27. Kumar L, Tanwar RK, Singh SP. Intracranial metastases from carcinoma cervix and review of literature. Gynecol Oncol. 1992. 46: 391-2
28. Lefkowitz D, Asconape J, Biller J. Intracranial metastases from carcinoma of the cervix. Southern Med J. 1983. 76: 519-21
29. Li H, Wu X, Cheng X. Advances in diagnosis and treatment of metastatic cervical cancer. J Gynecol Oncol. 2016. 27: e43-
30. Li N, Wang Y, Che S, Yang Y, Piao J, Liu S. HBXIP over expression as an independent biomarker for cervical cancer. Exp Mol Pathol. 2017. 102: 133-7
31. Mahmoud-Ahmed AS, Suh JH, Barnett GH, Webster KD, Kennedy AW. Tumor distribution and survival in six patients with brain metastases from cervical carcinoma. Gynecol Oncol. 2001. 81: 196-200
32. Marongiu A, Salvati M, D’Elia A, Arcella A, Giangaspero F, Esposito V. Single brain metastases from cervical carcinoma: Report of two cases and critical review of the literature. Neurol Sci. 2012. 33: 937-40
33. Matsunaga S, Shuto T, Sato M. Gamma knife surgery for metastatic brain tumors from gynecologic cancer. World Neurosurg. 2016. 89: 455-63
34. Menendez JY, Bauer DF, Shannon CN, Fiveash J, Markert JM. Stereotactic radiosurgical treatment of brain metastasis of primary tumors that rarely metastasize to the central nervous system. J Neurooncol. 2012. 109: 513-9
35. Nagar YS, Shah N, Rawat S, Kataria T. Intracranial metastases from adenocarcinoma of cervix: A case report. Int J Gynecol Cancer. 2005. 15: 561-3
36. Nasu K, Satoh T, Nishio S, Nagai Y, Ito K, Otsuki T. Clinicopathologic features of brain metastases from gynecologic malignancies: A retrospective study of 139 cases (KCOG-G1001s trial). Gynecol Oncol. 2013. 128: 198-203
37. Omari-Alaoui HE, Gaye PM, Kebdani T, El Ghazi E, Benjaafar N, Mansouri A. Cerebellous metastases in patients with uterine cervical cancer. Two cases reports and review of the literature. Cancer Radiother. 2003. 7: 317-20
38. Park SH, Ro DY, Park BJ, Kim YW, Kim TE, Jung JK. Brain metastasis from uterine cervical cancer. J Obstet Gynaecol Res. 2010. 36: 701-4
39. Peters P, Bandi H, Efendy J, Perez-Smith A, Olson S. Rapid growth of cervical cancer metastasis in the brain. J Clin Neurosci. 2010. 17: 1211-2
40. Pyeon SY, Park JY, Ulak R, Seol HJ, Lee JM. Isolated brain metastasis from uterine cervical cancer: A case report and review of literature. Eur J Gynaecol Oncol. 2015. 36: 602-4
41. Rahmathulla G, Toms SA, Weil RJ. The molecular biology of brain metastasis. J Oncol 2012. 2012. p.
42. Robinson JB, Morris M. Cervical carcinoma metastatic to the brain. Gynecol Oncol. 1997. 66: 324-6
43. Salvati M, Caroli E, Orlando ER, Nardone A, Frati A, Innocenzi G. Solitary brain metastases from uterus carcinoma: Report of three cases. J Neurooncol. 2004. 66: 175-8
44. Sato Y, Tanaka K, Kobayashi Y, Shibuya H, Nishigaya Y, Momomura M. Uterine cervical cancer with brain metastasis as the initial site of presentation. J Obstet Gynaecol Res. 2015. 41: 1145-8
45. Senapati SN, Samanta DR, Giri SK, Mohanty BK, Nayak CR. Carcinoma cervix with brain metastasis. J Indian Med Assoc. 1998. 96: 352-3
46. Setoodeh R, Hakam A, Shan Y. Cerebral metastasis of cervical cancer, report of two cases and review of the literature. Int J Clin Exp Pathol. 2012. 5: 710-4
47. Tajran D, Berek JS. Surgical resection of solitary brain metastasis from cervical cancer. Int J Gynecol Cancer. 2003. 13: 368-70
48. van Meir H, Kenter GG, Burggraaf J, Kroep JR, Welters MJ, Melief CJ. The need for improvement of the treatment of advanced and metastatic cervical cancer, the rationale for combined chemo-immunotherapy. Anticancer Agents Med Chem. 2014. 14: 190-203
49. Vitorino-Araujo JL, Veiga JC, Barboza VR, de Souza N, Mayrink D, Nadais RF. Scalp, skull and brain metastasis of squamous cell carcinoma of the cervix–a rare entity. Br J Neurosurg. 2013. 27: 519-20
50. Wuntkal R, Maheshwari A, Kerkar RA, Kane SV, Tongaonkar HB. Carcinoma of uterine cervix primarily presenting as carcinomatous meningitis: A case report. Aust N Z J Obstet Gynaecol. 2004. 44: 268-9
51. Zhang W, He W, Shi Y, Gu H, Li M, Liu Z. High expression of KIF20A is associated with poor overall survival and tumor progression in early-stage cervical squamous cell carcinoma. PLoS one. 2016. 11: e0167449-
52. Zhao M, Li Y, Wei X, Zhang Q, Jia H, Quan S. Negative immune factors might predominate local tumor immune status and promote carcinogenesis in cervical carcinoma. Virol J. 2017. 14: 5-
53. Ziainia T, Resnik E. Hemiballismus and brain metastases from squamous cell carcinoma of the cervix. Gynecol Oncol. 1999. 75: 289-92