- Department of Neurosurgery, Hospital Córdoba, Córdoba, Argentina
Correspondence Address:
Pablo E. Papalini
Department of Neurosurgery, Hospital Córdoba, Córdoba, Argentina
DOI:10.4103/sni.sni_363_16
Copyright: © 2017 Surgical Neurology International This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Pablo E. Papalini, Francisco R. Papalini. Cervicothoracic cutaneomeningospinal angiomatosis in adults (Cobb's syndrome): A case report of acute quadriparesis. 18-Jul-2017;8:147
How to cite this URL: Pablo E. Papalini, Francisco R. Papalini. Cervicothoracic cutaneomeningospinal angiomatosis in adults (Cobb's syndrome): A case report of acute quadriparesis. 18-Jul-2017;8:147. Available from: http://surgicalneurologyint.com/surgicalint-articles/cervicothoracic-cutaneomeningospinal-angiomatosis-in-adults-cobbs-syndrome-a-case-report-of-acute-quadriparesis/
Abstract
Background:Cutaneomeningospinal angiomatosis or Cobb syndrome is a rare, not well understood phacomatosis that features metameric cutaneous and spinal arteriovenous malformations (AVMs). The first case was described in Boston in 1915, and since then, few more cases have been reported in the English literature. No case was found to be from Argentina.
Case Description:The authors present a 16-year-old boy with acute quadriparesis and respiratory failure who was diagnosed as Cobb syndrome and treated with microsurgery alone with very good results.
Conclusion:Authors believe that microsurgery, alone or combined with embolization, should be the mainstay of treatment. They also acknowledge Harvey Cushing's contribution to the description of the syndrome and propose the syndrome to be renamed as Cobb–Cushing syndrome.
Keywords: Acute quadriparesis, Cobb syndrome, cutaneomeningospinal angiomatosis, Harvey Cushing, phacomatosis, spinal arteriovenous malformation
INTRODUCTION
The first case of a cutaneomeningospinal angiomatosis was described by Stanley Cobb in 1915,[
Very little information is present in the medical literature since then, and the syndrome remains in obscurity regarding its physiopathology, genetics, natural history, and even diagnosis criteria and optimal treatment modality. We present a 16-year-old boy with undiagnosed Cobb syndrome who developed acute chest and right arm pain followed by severe quadriparesis and respiratory failure; the patient recovered substantially after microsurgical treatment without embolization. This is the first case presented from an institution of Argentina. We agree with Maramatton et al. that the contribution of Harvey Cushing to the description of this rare syndrome should be acknowledged,[
CASE DESCRIPTION
History and presentation
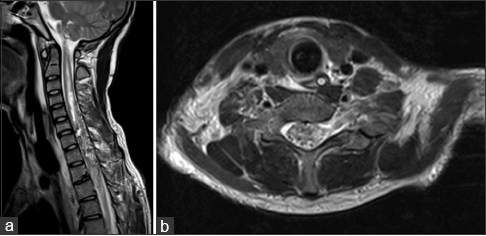
A 16-year-old boy was admitted to our center from another hospital to start plasmapheresis treatment, with the presumptive diagnosis of acute transverse myelitis. The symptoms started 8 days prior to his admittance in our institution, with sudden onset of severe right arm and chest pain, interpreted as a precordialgia secondary to ventricular arrhythmia, followed by progressive severe quadriparesis and respiratory failure. He was admitted few hours after the onset of symptoms to the regional local hospital and was sent to the Neurology Department at our hospital. Previous magnetic resonance imaging (MRI) findings were interpreted as a cevicodorsal acute transverse myelitis. The boy was previously healthy and had no medical history.
Examination
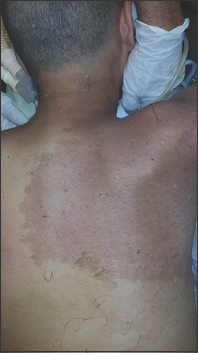
On examination, the patient presented flaccid paraplegia, with areflexia of the lower limbs, monoplegia of the right arm, and monoparesis of the left arm 2/5 proximal, 1/5 distal. He exhibited a complete sensory level at D4 and sphincter paralysis. He displayed a port-wine angioma in the right cervicodorsal and chest region [
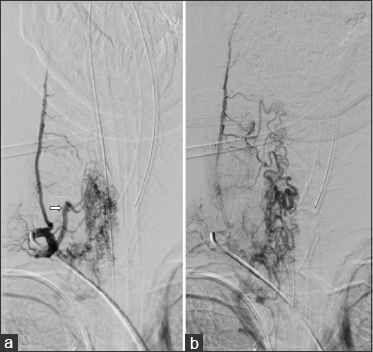
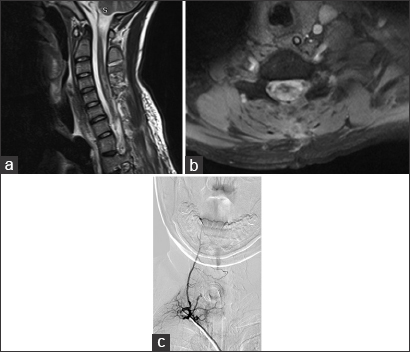
The patient had no improvement from the symptoms after a 4-day treatment with plasmapheresis, and was presented to our department, where the final diagnosis was made. We programmed surgery 9 days after arrival prior to obtaining a cervical DSA showing right-sided predominance of arterial feeding pedicles and several tortuous varicosal drain veins [
Figure 3
(a) Early arterial phase DSA in the right cervical region, showing a high-flow compact spinal AVM nidus with a main feeding trunk (white arrow) and multiple smaller ones. (b) Venous phase of the same angiogram, displaying multiple abnormal varicoceal draining veins extending well above the compact nidus in the cervical region
Intervention and postintervention course
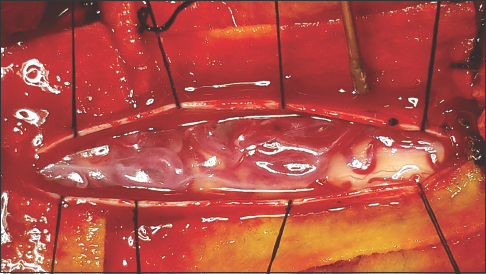
The patient was treated with a standard microsurgical procedure for spinal AVMs performed by the senior author (FRP) with no prior embolization. He was positioned supine, and C6-D1 bilateral laminectomy was performed. The dura was exposed and incised in the regular cefalocaudal fashion, showing underneath the varicosal draining shunted veins in the dorsal archnopial compartment [
In the subsequent control MRI and angiography, there was absence of lesion and significant lowering of the signal intensity above and below the lesion in the remaining spinal cord [
Figure 5
(a) Ten days postoperative sagittal MRI showing complete removal of the AVM and significant improvement of the hyperintensity in the cervical spinal cord [
At the 6-month follow-up after surgery, the patient continued rehabilitating in his home town in an outpatient basis, with both upper extremities improving functional capacity to the extent of being capable of self-feeding and performing daily activities. In the last follow-up 1 year after the surgery, the patient functions in a wheelchair, moves both arms with minor deficits, and can attend school.
DISCUSSION
After the first published description of this rare syndrome made by Stanley Cobb more than 100 years ago, there have been several adult and pediatric case reports and cases series in the English literature, with literature reviews reporting 18 adult cases for 2009 and 45 cases in 2012, along with one article in Chinese reporting a series of 61 cases.[
We believe that this syndrome, rare and often misdiagnosed, should be highly suspected in any patient presenting port-wine stains on the skin in a metameric disposition, and screening diagnostic spinal MRI should be warranted for both the patient and close family members. We also think that the optimal treatment modality is, as with most spinal AVMs, microsurgery (alone or combined with previous embolization) to both ligate and coagulate the feeding vessels and remove the mass effect upon the spinal cord, ideally before the bleeding occurs, with its devastating clinical outcome once the damage is established. We emphasize the role of microsurgery because it is the only treatment modality that offers (in selected cases of compact AVMs) complete removal of the malformation and can achieve very good results if diagnosis is followed by treatment in a timely manner. Further studies are needed to determine the best modality of treatment and better demarcate diagnostic criteria.
Finally, we agree with other authors opinion, that the contribution of Harvey Cushing should be acknowledged,[
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
Authors acknowledge the Neurosurgery Department of the Hospital Córdoba: Dr. Ricardo Olocco, Dr. Matías Berra, Dr. Emilio Mezzano, Dr. Javier Sánchez.
Author Queries via mail to: General Deheza 356 - 3 E - Córdoba X5000 - Argentina.
E-mail: pablo.papalini.bcn@gmail.com - frpapalini@gmail.com
References
1. Baraitser P, Shieff C. Cutaneomeningo-spinal angiomatosis: The syndrome of Cobb.A case report. Neuropediatrics. 1990. 21: 160-1
2. Cobb S. Haemangioma of the spinal cord: Associated with skin naevi of the same metamere. Ann Surg. 1915. 62: 641-9
3. Johnson WD, Petrie MM. Variety of spinal vascular pathology seen in adult Cobb syndrome. J Neurosurg Spine. 2009. 10: 430-5
4. Kissel P, Dureux B, Vinken PJ, Bruyn GW.editors. Cobb syndrome. Cutaneomeningospinal angiomatosis. The Phakomatoses. Handbook of Clinical Neurology. Amsterdam: North Holland Publishing; 1972. 14: 459-
5. Liang CH, Zhang HQ, Zhi XL, Zhang P, Li M, Ling F. Treatment of cutaneous vertebral medullary angiomatosis. Zhonghua Yi Xue Za Zhi. 2010. 90: 882-5
6. Linfante I, Capone FT, Dabus G, Gonzalez-Arias S, Lau PE, Samaniego EA. Spinal arteriovenous malformation associated with spinal metameric syndrome: A treatable cause of long-term paraplegia?. J Neurosurg Spine. 2012. 16: 408-13
7. Maramattom BV, Cohen-Gadol AA, Wijdicks EF, Kallmes D. Segmental cutaneous hemangioma and spinal arteriovenous malformation (Cobb syndrome).Case report and historical perspective. J Neurosurg Spine. 2005. 3: 249-52
8. Maramattom BV, Mercer RD, Rothner AD, Cook SA. The Cobb syndrome: Association with hereditary cutaneous hemangiomas. Cleve Clin Q. 1978. 45: 237-40
9. Matsui Y, Mineharu Y, Satow T, Takebe N, Takeuchi E, Saiki M. Coexistence of multiple cavernous angiomas in the spinal cord and skin: A unique case of Cobb syndrome. J Neurosurg Spine. 2014. 20: 142-7
10. Pal P, Ray S, Chakraborty S, Dey S, Talukdar A. Cobb syndrome: A rare cause of paraplegia. Ann Neurosci. 2015. 22: 191-3
11. Spetzler RF, Detwiller PW, Riina HA, Porter RW. Modified classification of spinal cord vascular lesions. J Neurosurg (Spine 2). 2002. 96: 145-56