- Neurosurgical Department, Klinikum Dortmund, Germany
- Department for Head and Neck Surgery, Klinikum Dortmund, Germany
- Department of Plastic Surgery, Bergmannsheil Bochum, Ruhr-University, Bochum, Germany
Correspondence Address:
Ali Harati
Department for Head and Neck Surgery, Klinikum Dortmund, Germany
DOI:10.4103/sni.sni_129_16
Copyright: © 2017 Surgical Neurology International This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Ali Harati, Kai-Michael Scheufler, Rolf Schultheiss, Albaraa Tonkal, Kamran Harati, Paul Oni, Thomas Deitmer. Clinical features, microsurgical treatment, and outcome of vestibular schwannoma with brainstem compression. 05-Apr-2017;8:45
How to cite this URL: Ali Harati, Kai-Michael Scheufler, Rolf Schultheiss, Albaraa Tonkal, Kamran Harati, Paul Oni, Thomas Deitmer. Clinical features, microsurgical treatment, and outcome of vestibular schwannoma with brainstem compression. 05-Apr-2017;8:45. Available from: http://surgicalneurologyint.com/surgicalint-articles/clinical-features-microsurgical-treatment-and-outcome-of-vestibular-schwannoma-with-brainstem-compression/
Abstract
Background:Presenting symptoms, treatment considerations, and outcome are strongly related to the extension of vestibular schwannomas (VS). The aim of the current retrospective study was to analyze the clinical features, microsurgical treatment, and outcome of VS with brainstem compression.
Methods:Forty-nine patients presented with VS (Hannover grading scale T4a or T4b) in our department. A subgroup analysis was performed among patients without (T4a) and with (T4b) compression and dislocation of the fourth ventricle.
Results:Patients with type T4b VS presented significantly more often with long tract signs/ataxia (P P P
Conclusions:VS with brainstem compression is frequently associated with hydrocephalus, ataxia, long tract signs, multiple cranial nerve disorders, and occasionally, signs of intracranial hypertension. Primary microsurgical resection is an appropriate management option for large VS.
Keywords: Cystic tumor, Hannover grading scale, hydrocephalus, microsurgery, vestibular schwannoma
INTRODUCTION
Vestibular schwannoma (VS), the most common tumor of the cerebellopontine angle (CPA), is a benign, slow-growing neoplasm. Presenting symptoms, treatment considerations, and outcome are strongly related to the size and extension of VS. Microsurgical treatment of large VS presents unique challenges. However, advances in microsurgical techniques, neuroanesthesia, and intensive care coupled with intraoperative neurophysiological monitoring, have led to remarkable improvements in clinical outcome.[
PATIENTS AND METHODS
Patients
Three hundred and forty patients with VS were treated in our institution between January 2004 and November 2015. Of these patients, 49 (22 women, 27 men) displayed brainstem compression on magnetic resonance imaging (MRI) and were included in the study. The mean age at presentation was 53 years (range: 18–77 years). Patients’ files and images were reviewed retrospectively. Follow-up data were collected throughout follow-up. The last mean follow-up time was 56 months (range: 4–202 months).
Clinical evaluation
All patients underwent complete perioperative neurological evaluation. Functional outcomes at final follow-up were assessed by a phone survey using the Karnofsky performance scale. Facial nerve function was assessed before and after surgery and at each follow-up using the House–Brackmann scale and categorized as good (HB I–II), fair (HB III–IV), and poor (HB V and VI). Pure tone audiograms were performed prior to surgery to assess the option of hearing preservation, as well as postoperatively in patients with serviceable hearing. Dysphagia and vocal cord function were evaluated to assess lower cranial nerve involvement. Evaluation of trigeminal nerve included motor and sensory function. Motor examination, including strength of all extremities, coordination, and gait, were examined routinely to assess brainstem and cerebellar affection. In addition, level of consciousness, orientation, memory, and other signs of elevated intracranial pressure (ICP) were evaluated. In selected cases (visual impairment, diplopia, nystagmus), ophthalmological examination was performed before and after surgery. Vestibular function was assessed by Frenzel glasses, testing of nystagmus during different manoeuvers, and video-nystagmography with caloric testing.
Radiologic evaluation
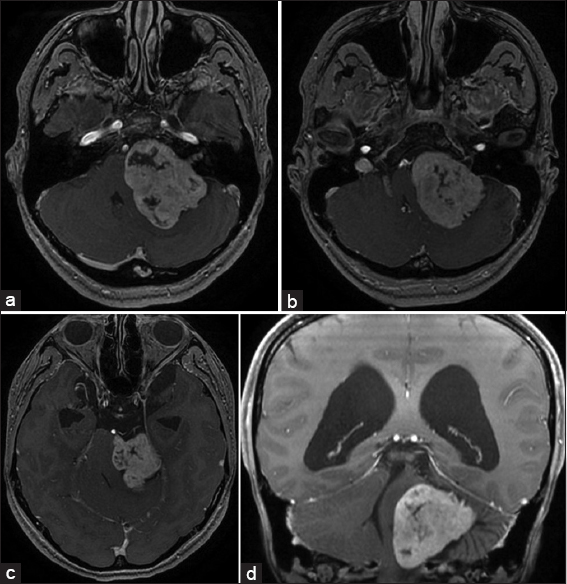
High-resolution bone window computed tomography (CT) studies, essential for visualizing the anatomy of the posterior semicircular canal and its vicinity to the posterior wall of the internal auditory canal and a high jugular bulb, were obtained before surgery. In addition, MRI was performed among all patients to assess tumor extension and maximal extrameatal diameter. The size of the tumor was determined based on linear planimetric measurements, and only the largest extrameatal diameter was used. T-2 weighted MR-imaging studies were used to assess hydrocephalus and tumor consistency (solid or cystic). Follow-up MRI was performed in all patients 3 months after the surgery to exclude residual tumor, and then every year to exclude recurrence. Residualcontrast enhancement along facial nerve or brainstem with a diameter exceeding 5 mm were indicative of subtotal resection (STR), whereas residual contrast-enhanced tissue measuring less than 5 mm was assumed to represent near-total resection (NTR). However, in the latter case, differentiation between residual tumor and scar tissue remains difficult.
Statistical analysis
Data were analyzed using the Statistical Package for the Social Sciences (SPSS) version 19 (SPSS, Inc., Chicago, IL). Categorical variables were compared using Fisher's exact two-tailed test and Pearson's χ2 test. Continuous variables were compared between groups with the Mann–Whitney U test.
RESULTS
Clinical and radiological presentation
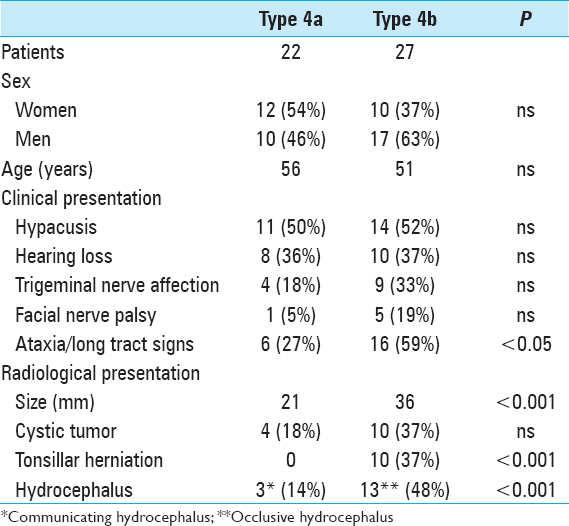
Prior to surgery, severe hypacusis was demonstrated in 26 patients (53%) whereas complete hearing loss had occurred in 17 patients (35%). Only 6 patients (11%) had only moderate hearing impairment (pure tone audiogram <30 db). Interestingly, complete hearing loss had occurred in 6 patients more than 5 years before the initial diagnosis of VS was made. Thirteen patients (27%) had trigeminal nerve involvement, and 2 of these patients had symptoms resembling trigeminal neuralgia. Six patients (12%) had facial nerve dysfunction, including moderate palsy (HB Grade II-III) in 4 patients and hemifacial spasm in 2 individuals. Abducens nerve palsy was present in 2 patients (4%). Gait ataxia and long tract signs were observed in 22 patients (45%), and acute hemiparesis was observed in 2 patients (4%).
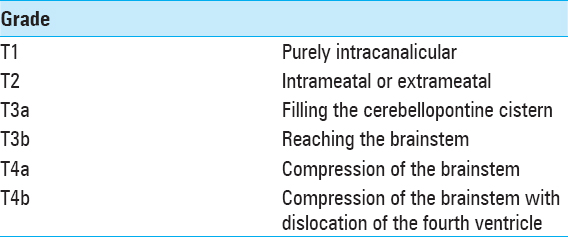
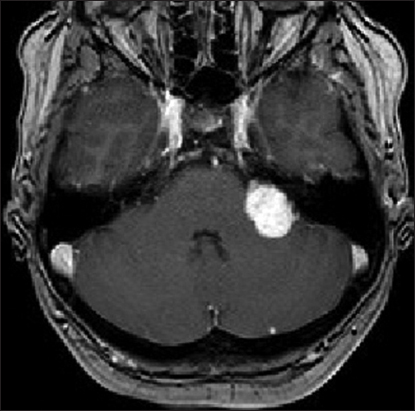
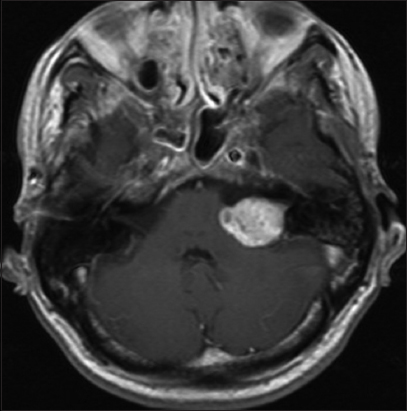
Signs of intracranial hypertension (severe headache, loss of orientation, nausea, vomiting, and/or papilledema) were present in 9 patients (18%). Mean tumor size was 30 mm (range: 18–55 mm). Radiological signs of hydrocephalus were observed in 16 patients (33%), whereas 10 patients (20%) had signs of tonsillar herniation. Both of these signs were closely associated with type T4b VS (P > 0.01). Cystic changes were more common in type 4b tumors than type 4a tumors, though the difference did not reach statistical significance [
Operative procedure and postoperative course
Surgery was performed through a retrosigmoid craniotomy, with the patient positioned in the park bench position. Intraoperative monitoring of facial nerve function was used in all cases. We performed standard monitoring techniques such as free-running facial nerve electromyography (EMG), direct electrical stimulation EMG, and in recent years, facial motor evoked potential (FMEP). In case of servicable hearing, we additionally performed intraoperative brainstem auditory evoked potential (BAEP) monitoring.
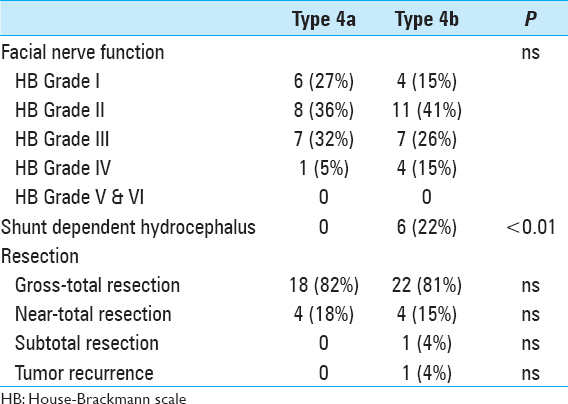
Mean operation time was 6.5 hours (range: 2.5–12 hours). Gross total resection was achieved in 41 patients (84%), near-total resection was achieved in 7 patients (14%), and subtotal resection was performed in 1 patient in whom the tumor had infiltrated the arachnoid of the brainstem; staged resection was performed in another patient. Eight patients required a perioperative ventricular drain due to acute occlusive hydrocephalus. Severe complications were observed in 3 patients (6%). Subarachnoid hemorrhage occurred in a 68-year-old woman. A ventricular drain was inserted and the patient went on to full recovery. In a 47-year-old man, a ventricular drain caused a right frontal lobe hematoma with cerebral herniation prompting immediate surgical revision. A 50-year-old woman died from severe postoperative posterior fossa hematoma caused by a severe platelet disorder, which was not diagnosed before surgery. Subsequently, a bleeding time test (Ivy method) was included in our routine preoperative workup. Twenty-seven patients (63%) displayed temporary worsening of facial nerve function after surgery. In 14 patients, a combined upper eye lid loading and lower lid canthopexy were performed to provide symptomatic relief from corneal exposure with a reasonable cosmetic appearance. At latest follow-up, no patient had poor facial nerve outcome (HB V–VI), 43 patients (69%) had favorable facial nerve function (HB I–II), and 19 patients (31%) had fair facial nerve function (HB III–IV) [
Long-term outcome
Patients were followed clinically for a mean of 54 months (range: 4–124 months). No patient was lost or died during follow-up. Tumor regrowth occurred in the patient who had undergone subtotal resection. He was referred to stereotactic radiation therapy. Overall recovery assessment based on the Karnofsky performance scale (KPS) during long-term follow-up yielded a KPS scores of 90% in 18 patients, 80% in 20 patients, and 70% in 7 patients [
DISCUSSION
The management of large VS is challenging. Patients usually present with multiple cranial nerve deficits and signs of brainstem compression or intracranial hypertension. Our study indicates that even in VS with brainstem compression, complete tumor removal with favorable outcome is feasible.[
Clinical considerations
VS is commonly classified according to the maximum extrameatal tumor diameter.[
VS is diagnosed more frequently and in earlier stages nowadays due to increased utilization of cranial MRI for a variety of indications. However, in many countries, large and giant VS are still frequently encountered at initial diagnosis because of a less developed healthcare system or other reasons.[
Treatment
Treatment options for VS generally include observation, microsurgical resection, and radiosurgery. A wait-and-scan policy is generally not recommended for large VS with brainstem compression because of potentially life-threatening complications caused by further tumor growth. Radiosurgery does not induce immediate relief from tumor mass effect from large VS, and tumor volume may increase as a result of radiation-induced swelling.[
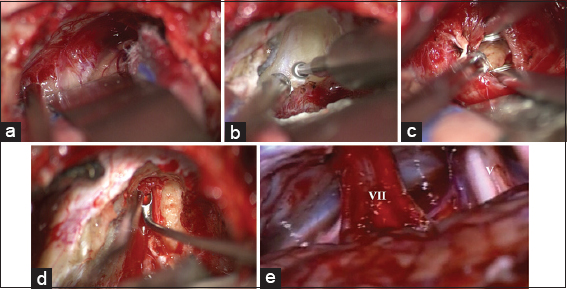
Figure 5
(a-d) Intraoperative image of a vestibular schwannoma (T4b) exposed through a retrosigmoid approach on the right side in park bench position. (a) Tumor exposure; (b) Drilling of internal acoustic canal in close relationship to the jugular bulb*; (c) Tumor debulking; (d) Removal of the intrameatal tumor; (e) The cerebellopontine angle after tumor removal
A major factor influencing postoperative quality of life is facial nerve dysfunction. Ansari et al. in their systematic review reported that the translabyrinthine approach was associated with inferior facial nerve function. However, their analysis might have been confounded by the inclusion of larger VS approached through the translabyrinthine route.[
Because a randomized trial comparing the different operative approaches is not feasible, there is no general recommendation regarding the surgical approach for VS. It is generally accepted that surgeon's preference and experience may be the key determinant of the surgical approach. Because VS grows slowly, the facial nerve becomes stretched out flat along the tumor surface in large VS. The arachnoid dissection plane becomes obscured, and microsurgical dissection therefore becomes very challenging. Following tumor resection, a collage sponge coated with fibrinogen was used to stabilize the thinned facial nerve in its course through the CPA in most patients of our series. Microsurgical skills and experience of the surgeon influence postoperative facial nerve function.[
The implementation of intraoperative neuromonitoring during resection of VS is associated with improvements in functional preservation of the cranial nerves. Among these methods, the most frequently used are direct electrical stimulation EMG, free-running EMG, and FMEP for the facial nerve and BAEP together with cochlear nerve action potentials (CNAP) for the vestibulocochlear nerve.[
Some authors advocate subtotal resection to reduce the risk of hearing loss and facial nerve dysfunction. In our series, subtotal resection was performed in one patient only, who later displayed tumor recurrence. Incomplete resection is associated with high recurrence rates (up to 39%).[
CONCLUSIONS
We demonstrate the distinct clinical findings of VS with brainstem compression, noting that these tumors are frequently associated with hydrocephalus, ataxia, long tract signs, multiple cranial nerve disorders, and in some cases with signs of intracranial hypertension. Primary microsurgical resection (gross total or near total) appears to be the first-line management option of choice for large VS.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Anaizi AN, Gantwerker EA, Pensak ML, Theodosopoulos PV. Facial nerve preservation surgery for koos grade 3 and 4 vestibular schwannomas. Neurosurgery. 2014. 75: 671-5
2. Ansari SF, Terry C, Cohen-Gadol AA. Surgery for vestibular schwannomas: A systematic review of complications by approach. Neurosurg Focus. 2012. 33: E14-
3. Bambakidis NC, Lo SS, Selman WR. Large vestibular schwannomas. J Neurosurg. 2011. 115: 894-5
4. Bandlish D, Biswas N, Deb S. Staging in giant vestibular schwannoma surgery: A two consecutive day technique for complete resection in basic neurosurgical setups. J Neurosci Rural Pract. 2014. 5: 225-30
5. Ben Ammar M, Piccirillo E, Topsakal V, Taibah A, Sanna M. Surgical results and technical refinements in translabyrinthine excision of vestibular schwannomas: The Gruppo Otologico experience. Neurosurgery. 2012. 70: 1481-91
6. Carlson ML, Van Abel KM, Driscoll CL, Neff BA, Beatty CW, Lane JI. Magnetic resonance imaging surveillance following vestibular schwannoma resection. Laryngoscope. 2012. 122: 378-88
7. Chamoun R, MacDonald J, Shelton C, Couldwell WT. Surgical approaches for resection of vestibular schwannomas: Translabyrinthine, retrosigmoid, and middle fossa approaches. Neurosurg Focus. 2012. 33: E9-
8. Chopra R, Fergie N, Mehta D, Liew L. The middle cranial fossa approach: An anatomical study. Surg Radiol Anat. 2003. 24: 348-51
9. Ciric I, Zhao J-C, Rosenblatt S, Wiet R, O’Shaughnessy B. Suboccipital retrosigmoid approach for removal of vestibular schwannomas: Facial nerve function and hearing preservation. Neurosurgery. 2005. 56: 560-70
10. Cole T, Veeravagu A, Zhang M, Azad T, Swinney C, Li GH. Retrosigmoid Versus Translabyrinthine Approach for Acoustic Neuroma Resection: An Assessment of Complications and Payments in a Longitudinal Administrative Database. Cureus. 2015. 7: e369-
11. Day JD, Chen DA, Arriaga M. Translabyrinthine approach for acoustic neuroma. Neurosurgery. 2004. 54: 391-5
12. Di Maio S, Malebranche AD, Westerberg B, Akagami R. Hearing preservation after microsurgical resection of large vestibular schwannomas. Neurosurgery. 2011. 68: 632-40
13. Fundová P, Charabi S, Tos M, Thomsen J. Cystic vestibular schwannoma: Surgical outcome. J Laryngol Otol. 2000. 114: 935-9
14. Gerganov VM, Giordano M, Samii A, Samii M. Surgical treatment of patients with vestibular schwannomas after failed previous radiosurgery. J Neurosurg. 2012. 116: 713-20
15. Gerganov VM, Pirayesh A, Nouri M, Hore N, Luedemann WO, Oi S. Hydrocephalus associated with vestibular schwannomas: Management options and factors predicting the outcome. J Neurosurg. 2011. 114: 1209-15
16. Gharabaghi A, Samii A, Koerbel A, Rosahl SK, Tatagiba M, Samii M. Preservation of function in vestibular schwannoma surgery. Neurosurgery. 2007. 60: ONS124-7
17. Gurgel RK, Dogru S, Amdur RL, Monfared A. Facial nerve outcomes after surgery for large vestibular schwannomas: Do surgical approach and extent of resection matter?. Neurosurg Focus. 2012. 33: E16-
18. Iwai Y, Ishibashi K, Watanabe Y, Uemura G, Yamanaka K. Functional Preservation After Planned Partial Resection Followed by Gamma Knife Radiosurgery for Large Vestibular Schwannomas. World Neurosurg. 2015. 84: 292-300
19. Jain VK, Mehrotra N, Sahu RN, Behari S, Banerji D, Chhabra DK. Surgery of vestibular schwannomas: An institutional experience. Neurol India. 2005. 53: 41-5
20. Jian BJ, Sughrue ME, Kaur R, Rutkowski MJ, Kane AJ, Kaur G. Implications of cystic features in vestibular schwannomas of patients undergoing microsurgical resection. Neurosurgery. 2011. 68: 874-80
21. Kanzaki J, Tos M, Sanna M, Moffat DA, Monsell EM, Berliner KI. New and modified reporting systems from the consensus meeting on systems for reporting results in vestibular schwannoma. Otol Neurotol. 2003. 24: 642-8
22. Koos WT, Day JD, Matula C, Levy DI. Neurotopographic considerations in the microsurgical treatment of small acoustic neurinomas. J Neurosurg. 1998. 88: 506-12
23. Lanman TH, Brackmann DE, Hitselberger WE, Subin B. Report of 190 consecutive cases of large acoustic tumors (vestibular schwannoma) removed via the translabyrinthine approach. J Neurosurg. 1999. 90: 617-23
24. Mamikoglu B, Wiet RJ, Esquivel CR. Translabyrinthine approach for the management of large and giant vestibular schwannomas. Otol Neurotol. 2002. 23: 224-7
25. Matthies C, Samii M. Management of 1000 vestibular schwannomas (acoustic neuromas): Clinical presentation. Neurosurgery. 1997. 40: 1-9
26. Matthies C, Samii M, Krebs S. Management of vestibular schwannomas (acoustic neuromas): Radiological features in 202 cases--their value for diagnosis and their predictive importance. Neurosurgery. 1997. 40: 469-81
27. Mehrotra N, Behari S, Pal L, Banerji D, Sahu RN, Jain VK. Giant vestibular schwannomas: Focusing on the differences between the solid and the cystic variants. Br J Neurosurg. 2008. 22: 550-6
28. Monfared A, Corrales E, Theodosopoulos P, Blevins NH, Oghalai JS, Selesnick SH. Facial Nerve Outcome and Tumor Control Rate as a Function of Degree of Resection in Treatment of Large Acoustic Neuromas: Preliminary Report of the Acoustic Neuroma Subtotal Resection Study. Neurosurgery. 2016. 79: 194-203
29. Nagano O, Higuchi Y, Serizawa T, Ono J, Matsuda S, Yamakami I. Transient expansion of vestibular schwannoma following stereotactic radiosurgery. J Neurosurg. 2008. 109: 811-6
30. Nickele CM, Akture E, Gubbels SP, Başkaya MK. A stepwise illustration of the translabyrinthine approach to a large cystic vestibular schwannoma. Neurosurg Focus. 2012. 33: E11-
31. Pai I, Bowman J, Thomas N, Kitchen N, Strong A, Obholzer R. Management of large and giant vestibular schwannomas. Skull Base. 2011. 21: 379-84
32. Park CE, Park BJ, Lim YJ, Yeo SG. Functional outcomes in retrosigmoid approach microsurgery and gamma knife stereotactic radiosurgery in vestibular schwannoma. Eur Arch Otorhinolaryngol. 2011. 268: 955-9
33. Patni AH, Kartush JM. Staged resection of large acoustic neuromas. Otolaryngol Head Neck Surg. 2005. 132: 11-9
34. Raftopoulos C, Abu Serieh B, Duprez T, Docquier MA, Guérit JM. Microsurgical results with large vestibular schwannomas with preservation of facial and cochlear nerve function as the primary aim. Acta Neurochir. 2005. 147: 697-706
35. Ramina R, Coelho Neto M, Bordignon KC, Mattei T, Clemente R, Pires Aguiar PH. Treatment of large and giant residual and recurrent vestibular schwannomas. Skull Base. 2007. 17: 109-17
36. Raslan AM, Liu JK, McMenomey SO, Delashaw JB. Staged resection of large vestibular schwannomas. J Neurosurg. 2012. 116: 1126-33
37. Rhoton AL. The cerebellopontine angle and posterior fossa cranial nerves by the retrosigmoid approach. Neurosurgery. 2000. 47: S93-129
38. Samii M, Matthies C. Hearing preservation in acoustic tumour surgery. Adv Tech Stand Neurosurg. 1995. 22: 343-73
39. Samii M, Gerganov VM, Samii A. Functional outcome after complete surgical removal of giant vestibular schwannomas. J Neurosurg. 2010. 112: 860-7
40. Samii M, Gerganov V, Samii A. Improved preservation of hearing and facial nerve function in vestibular schwannoma surgery via the retrosigmoid approach in a series of 200 patients. J Neurosurg. 2006. 105: 527-35
41. Silva J, Cerejo A, Duarte F, Silveira F, Vaz R. Surgical removal of giant acoustic neuromas. World Neurosurg. 2012. 77: 731-5
42. Sinha S, Sharma BS. Cystic acoustic neuromas: Surgical outcome in a series of 58 patients. J Clin Neurosci. 2008. 15: 511-5
43. Springborg JB, Fugleholm K, Poulsgaard L, Cayé-Thomasen P, Thomsen J, Stangerup S-E. Outcome after translabyrinthine surgery for vestibular schwannomas: Report on 1244 patients. J Neurol Surg B Skull Base. 2012. 73: 168-74
44. van de Langenberg R, Hanssens PEJ, van Overbeeke JJ, Verheul JB, Nelemans PJ, de Bondt B-J. Management of large vestibular schwannoma. Part I. Planned subtotal resection followed by Gamma Knife surgery: Radiological and clinical aspects. J Neurosurg. 2011. 115: 875-84
45. van de Langenberg R, Hanssens PEJ, Verheul JB, van Overbeeke JJ, Nelemans PJ, Dohmen AJC. Management of large vestibular schwannoma. Part II. Primary Gamma Knife surgery: Radiological and clinical aspects. J Neurosurg. 2011. 115: 885-93
46. Wang M, Jia D, Shen J, Zhang J, Li G. Facial nerve function after large cystic vestibular schwannomas surgery via the retrosigmoid approach. Turk Neurosurg. 2013. 23: 161-9
47. Wanibuchi M, Fukushima T, McElveen JT, Friedman AH. Hearing preservation in surgery for large vestibular schwannomas. J Neurosurg. 2009. 111: 845-54
48. Wiet RJ, Mamikoglu B, Odom L, Hoistad DL. Long-term results of the first 500 cases of acoustic neuroma surgery. Otolaryngol Head Neck Surg. 2001. 124: 645-51
49. Yamakami I, Uchino Y, Kobayashi E, Yamaura A, Oka N. Removal of large acoustic neurinomas (vestibular schwannomas) by the retrosigmoid approach with no mortality and minimal morbidity. J Neurol Neurosurg Psychiatr. 2004. 75: 453-8
50. Yamakami I, Ito S, Higuchi Y. Retrosigmoid removal of small acoustic neuroma: Curative tumor removal with preservation of function. J Neurosurg. 2014. 121: 554-63
51. Yang J, Grayeli AB, Barylyak R, Elgarem H. Functional outcome of retrosigmoid approach in vestibular schwannoma surgery. Acta Otolaryngol. 2008. 128: 881-6
52. Youssef AS, Downes AE. Intraoperative neurophysiological monitoring in vestibular schwannoma surgery: Advances and clinical implications. Neurosurg Focus. 2009. 27: 3-6
53. Zhang Z, Wang Z, Huang Q, Yang J, Wu H. Removal of large or giant sporadic vestibular schwannomas via translabyrinthine approach: A report of 115 cases. J Otorhinolaryngol Relat Spec. 2012. 74: 271-7