- Department of Neurosurgery, Maastricht University Medical Center, Maastricht, The Netherlands
- Department of Neurology, Maastricht University Medical Center, Maastricht, The Netherlands
Correspondence Address:
Pieter L. Kubben
Department of Neurosurgery, Maastricht University Medical Center, Maastricht, The Netherlands
DOI:10.4103/sni.sni_234_18
Copyright: © 2018 Surgical Neurology International This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.How to cite this article: Aurélie Degeneffe, Mark L. Kuijf, Linda Ackermans, Yasin Temel, Pieter L. Kubben. Comparing deep brain stimulation in the ventral intermediate nucleus versus the posterior subthalamic area in essential tremor patients. 04-Dec-2018;9:244
How to cite this URL: Aurélie Degeneffe, Mark L. Kuijf, Linda Ackermans, Yasin Temel, Pieter L. Kubben. Comparing deep brain stimulation in the ventral intermediate nucleus versus the posterior subthalamic area in essential tremor patients. 04-Dec-2018;9:244. Available from: http://surgicalneurologyint.com/surgicalint-articles/9105/
Abstract
Background:The ventral intermediate nucleus (VIM) is the most commonly used target for deep brain stimulation (DBS) in patients with essential tremor (ET). Recent evidence suggests that the posterior subthalamic area (PSA) might be a better target for tremor reduction. We compared the outcome of VIM DBS with PSA DBS in our cohort of patients.
Methods:Overall, 19 ET patients with bilateral DBS were included in this retrospective study, with a total of 38 electrodes (12 located in the VIM, 12 in the PSA, and 14 in an intermediate area). The outcome was measured using the essential tremor rating scale (ETRS), the glass scale and the quality of life in essential tremor questionnaire (QUEST).
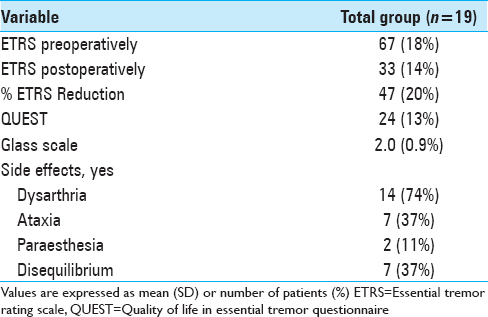
Results:Unilateral tremor-scores with items 5–6 (tremor of the upper extremity), 8–9 (tremor of the lower extremity), and 11-14 (hand function) from the ETRS showed a 63% tremor reduction in the VIM group, 47% tremor reduction in the PSA group, and 67% tremor reduction in the intermediate group after a mean follow-up of 1.6 years. After a mean follow-up of 5.8 years, there was a tremor reduction of 50%, 34%, and 45%, respectively. In our series, side effects such as dysarthria (75%), ataxia and disequilibrium (40%), and paraesthesia (15%) were assessed.
Conclusions:All aforementioned anatomical target areas are effective in reducing tremor, although no superior reduction was found with PSA stimulation. Because of intraindividual differences between left and right hemisphere regarding the stimulated anatomical target, no conclusions can be drawn regarding differences in side effects.
Keywords: Deep brain stimulation, essential tremor, posterior subthalamic area, ventral intermediate nucleus
INTRODUCTION
Formerly considered a benign aspect of aging, essential tremor (ET) is now acknowledged as the most common movement disorder.[
MATERIALS AND METHODS
Study design
We conducted a retrospective study evaluating all ET patients operated with DBS in our center between 2003 and 2016. The Medical Ethical Research Committee at the Maastricht University Medical Center approved the study and informed consent was obtained according to the Declaration of Helsinki.
Surgical procedure
The surgical procedure at our center has previously been described in detail by Kocabicak.[
Evaluation of electrode position
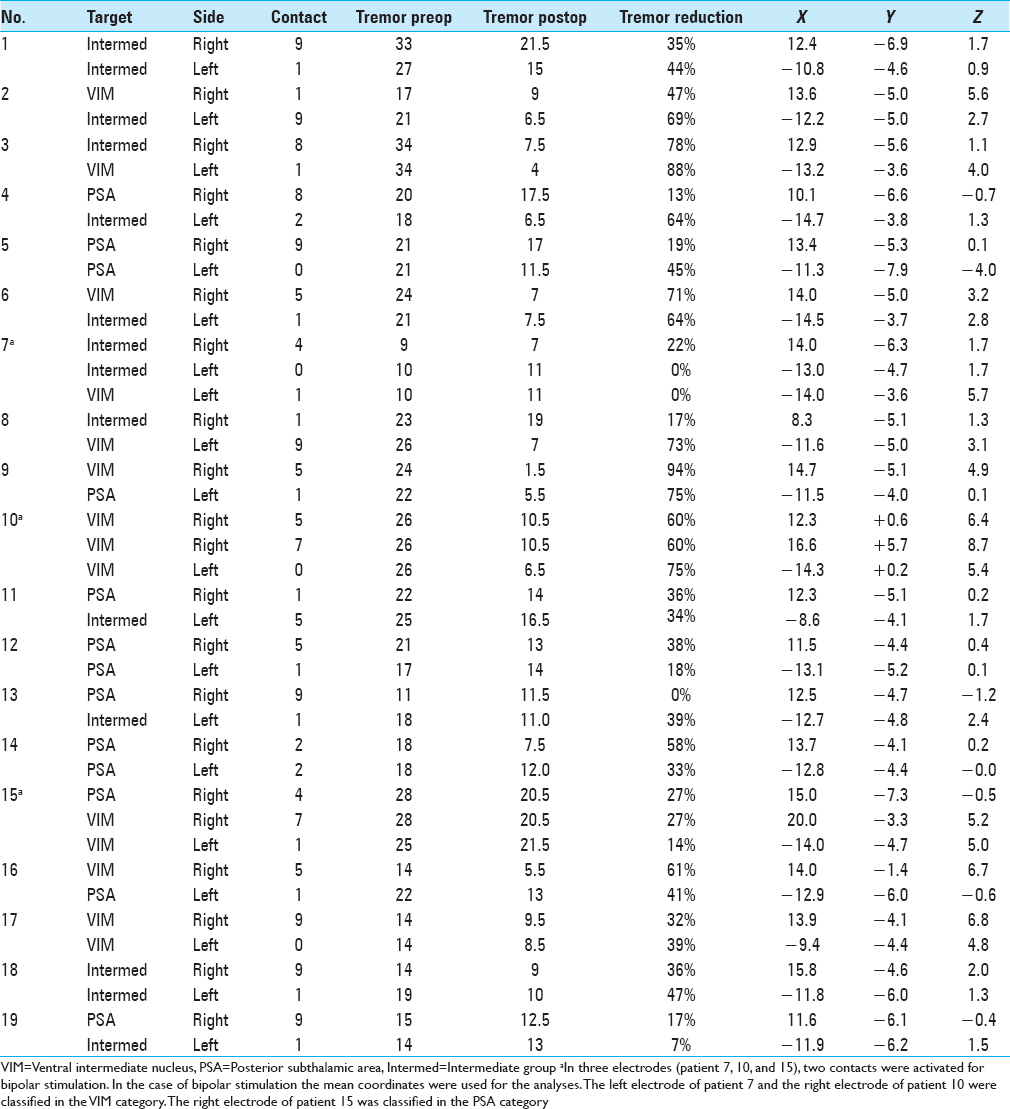
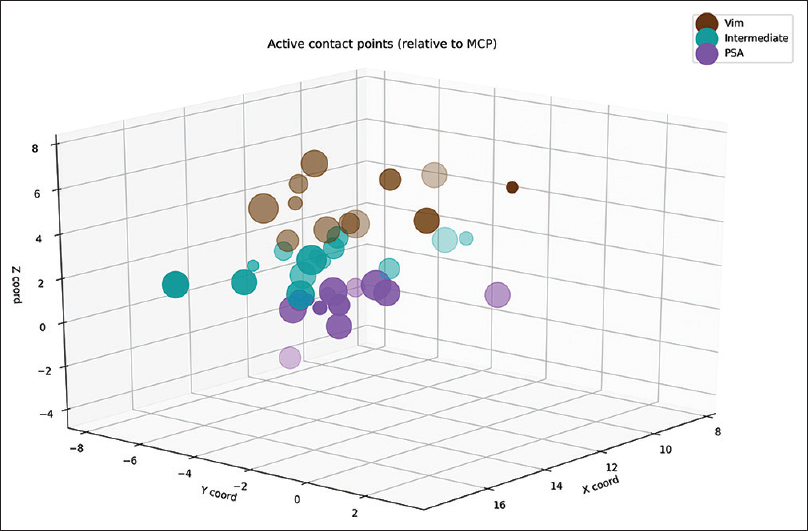
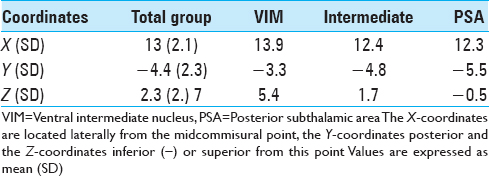
Postoperatively, we determined the coordinates of the middle of the active contact relative to the midcommissural point (MCP). We assessed these coordinates based on coregistering a preoperative MRI and a postoperative CT using the iPlan Stereotaxy software (Brainlab AG, Germany). In two cases, we used a postoperative MRI scan because no CT scan was available. Measurements were performed by the first author and randomly double-checked by the last author. In addition, we measured the width of the third ventricle at the height of the MCP. Based on the coordinates relative to the MCP, in particular the z-axis, the DBS targets were divided into three categories. If the coordinate was ≥3 mm above the z-axis, it was classified as VIM. If the coordinate was at or below the z-axis it was classified as PSA. If the contact was between 0 and 3 mm above the z-axis, it was classified as intermediate. We introduced this third category because tissue activation from contact points in this area may occur in both the VIM and the PSA, and categorizing the anatomical target as one of these could lead to incorrect conclusions.
Tremor evaluation
We systematically contacted all included patients for tremor assessment using the ETRS[
Statistics
Statistical analysis was performed using SPSS Statistics version 23 (IBM Corporation, Armonk, NY). Evaluation was performed for each single electrode, corresponding with the tremor on the contralateral side. A mixed models analysis was used to compensate for this repeated measurement within one subject. Mixed models analysis was also used to detect differences in stimulation parameters between the three groups and to detect a possible correlation between stimulation parameters and outcome. A value of P ≤ 0.05 was defined as the level of statistical significance.
RESULTS
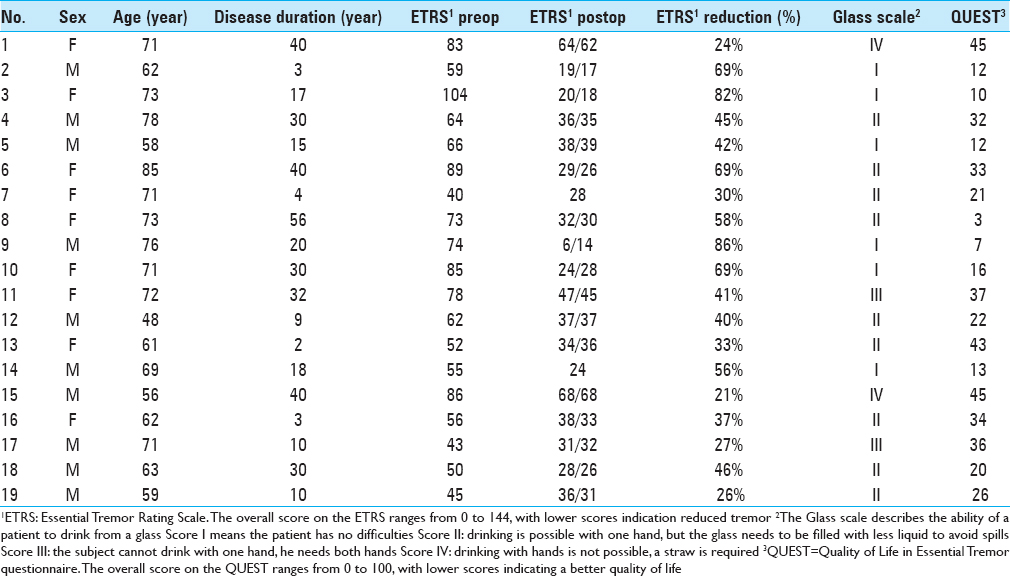
We analyzed 38 DBS electrodes in 19 patients. The mean age at implantation was 67 ± 9 years. The mean disease duration was 22 ± 16 years. Overall, 12 contact locations were classified as VIM, 12 contact locations as PSA, and 14 contact locations as intermediate. Patient characteristics are presented in
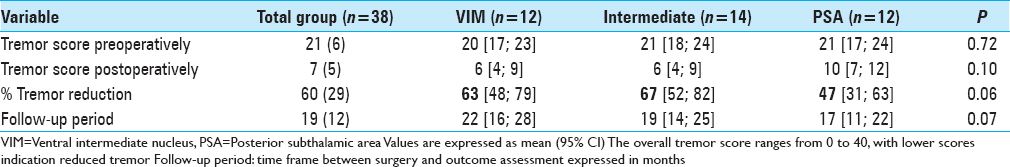
Clinical outcome
The overall postoperative ETRS reduction for all patients is 33 (47%) with a mean postoperative ETRS of 33 [
After a mean follow-up duration of 1 year and 7 months, there was a 63% tremor reduction in the VIM group, 67% tremor reduction in the intermediate group, and 47% tremor reduction in the PSA group, P = 0.059 [
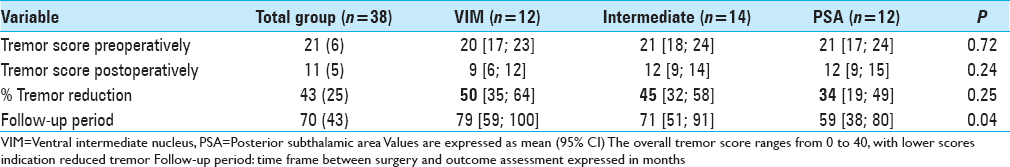
Regarding the tremor reduction in the subgroups, there was a 50% reduction in the VIM group, 45% tremor reduction in the intermediate group and 34%tremor reduction in the PSA group, P = 0.250 [
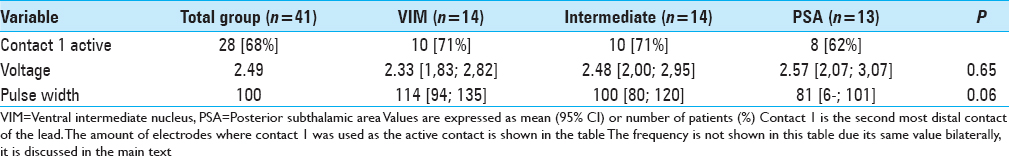
Stimulation parameters
Within the total group of electrodes, the second most distal contact (by convention named contact 1) was active in 68%. In three patients, two contacts were activated unilaterally for bipolar stimulation [Tables
DISCUSSION
In contrast to other studies, we did not find a statistical significant difference in tremor reduction between the VIM and PSA group. The results from this study suggest a better tremor reduction in the VIM group (50%) compared with the PSA group (34%). Regarding the side effects, there is high prevalence of dysarthria (74%). It is unclear whether this is due to the effect of DBS or the natural progression of the disease, as we did not obtain approval by the medical ethical committee to turn off stimulation for this study. There are no data available concerning the preoperative Glass scale, so we cannot conclude whether there is a functional improvement or not. There are still four patients with a glass scale of III or IV; the other patients have little functional limitation.
The PSA, including the zona incerta (ZI) and the prelemniscal radiation (Raprl), contains the dentato-rubro thalamic tract (DRTT), also called the cerebellothalamic tract, which is the main fiber bundle that forms the superior cerebellar peduncle. The superior cerebellar peduncle constitutes one of the largest efferent connections of the cerebellum. In the PSA, the fibers are very densely packed before they fan out to enter the ventralis oralis posterior or VIM region in the thalamus.[
In this study, postoperative assessment of electrode location was mainly done by coregistration of preoperative MRI to a postoperative CT, which has been validated up to an accuracy of 1–2 mm.[
Although no firm conclusions can be drawn from this retrospective analysis, there are noteworthy aspects from this study that feed the discussion on target selection for ET. First, stimulation of the PSA did not result in a better tremor control in this comparable cohort of ET patients. In fact, a trend toward better tremor control was found in the intermediate and VIM group compared with the PSA for which several explanations should be considered. For example, in the PSA group, there were less electrodes that used the nearest deepest contact, which suggests that perhaps by chance a nonoptimal position of the electrode was achieved in this group. In addition, stimulation parameters in the VIM group differed considerably with respect to the pulse-width that was applied, although we could not demonstrate any significant differences due to the low number of patients. The higher pulse-width also explains the somewhat higher voltage applied in the PSA group that was required to achieve a similar volume of tissue activation. This shift toward using lower pulse-widths in the PSA group may be a reflection of changes within the clinical DBS team and clinicians involved in programing the patients during the study period. In more recent years, the programing of DBS patients is performed by neurologists at our institution whereas in the earlier years of DBS implantation programing was usually done by the neurosurgical team. This also illustrates the main limitation of this study as the retrospective design compares not only patient specific differences but also changes in clinical practice. This taken into consideration, the observed reduction of tremor in this series of patients receiving PSA stimulation does not really look promising toward changing anatomical targets for ET using current imaging techniques.
CONCLUSION
Both VIM and PSA DBS are effective in reducing tremor in ET patients; however, based on our results, we cannot conclude whether one target is superior to the other one. Prospective research in a larger population is needed for stronger conclusions, and preferably includes fiber tracking of the DRTT for individual targeting and volume of tissue activation modeling for a more representative impression of the actual brain areas involved in DBS.
Competing interests
The authors declare that they have no competing interests.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
The authors would like to thank gratefully Nicole Bakker for her invaluable clinical assistance and Bjorn Winkens for statistical consultation.
References
1. Bally JF, Vargas M-I, Horvath J, Fleury V, Burkhard P, Momjian S. Localization of deep brain stimulation contacts using corticospinal/corticobulbar tracts stimulation. Front Neurol. 2017. 8: 1-7
2. Barbe MT, Liebhart L, Runge M, Deyng J, Florin E, Wojtecki L. Deep brain stimulation of the ventral intermediate nucleus in patients with essential tremor: Stimulation below intercommissural line is more efficient but equally effective as stimulation above. Exp Neurol. 2011. 230: 131-7
3. Benabid AL, Pollak P, Gao D, Hoffmann D, Limousin P, Gay E. Chronic electrical stimulation of the ventralis intermedius nucleus of the thalamus as a treatment of movement disorders. J Neurosurg. 1996. 84: 203-14
4. Benabid AL, Pollak P, Hoffmann D, Gervason C, Hommel M, Perret JE. Long-term suppression of tremor by chronic stimulation of the ventral intermediate thalamic nucleus. Lancet. 1991. 337: 403-6
5. Blomstedt P, Fytagoridis A, Tisch S. Deep brain stimulation of the posterior subthalamic area in the treatment of tremor. Acta Neurochir (Wien). 2009. 151: 31-6
6. Blomstedt P, Sandvik U, Tisch S. Deep brain stimulation in the posterior subthalamic area in the treatment of essential tremor. Mov Disord. 2010. 25: 1350-6
7. Chaturvedi A, Luján JL, McIntyre CC. Artificial neural network based characterization of the volume of tissue activated during deep brain stimulation. J Neural Eng. 2013. 10: 056023-
8. Coenen VA, Mädler B, Schiffbauer H, Urbach H, Allert N. Individual fiber anatomy of the subthalamic region revealed with diffusion tensor imaging: A concept to identify the deep brain stimulation target for tremor suppression. Neurosurgery. 2011. 68: 1069-76
9. Deuschl G, Raethjen J, Hellriegel H, Deuschl G, Raethjen J, Hellriegel H. Treatment of patients with essential tremor. Rev Lancet Neurol. 2011. 10: 148-61
10. Elble R, Bain P, João Forjaz M, Haubenberger D, Testa C, Goetz CG. Task force report: Scales for screening and evaluating tremor: Critique and recommendations. Mov Disord. 2013. 28: 1793-800
11. Elias WJ, Shah BB. Tremor. Jama. 2014. 311: 948-
12. Flora ED, Perera CL, Cameron AL, Maddern GJ. Deep brain stimulation for essential tremor: A systematic review. Mov Disord. 2010. 25: 1550-9
13. Fytagoridis A, Åström M, Wårdell K, Blomstedt P. Stimulation-induced side effects in the posterior subthalamic area: Distribution, characteristics and visualization. Clin Neurol Neurosurg. 2013. 115: 65-71
14. Gironell A, Martínez-Corral M, Pagonabarraga J, Kulisevsky J. The Glass scale: A simple tool to determine severity in essential tremor. Park Relat Disord. 2010. 16: 412-4
15. Gorman RLO, Jarosz JM, Samuel M, Clough C, Selway RP, Ashkan K. CT/MR Image fusion in the postoperative assessment of electrodes implanted for deep brain stimulation. Stereotact Funct Neurosurg. 2009. 87: 205-10
16. Groppa S, Herzog J, Falk D, Riedel C, Deuschl G, Volkmann J. Physiological and anatomical decomposition of subthalamic neurostimulation effects in essential tremor. Brain. 2014. 137: 109-21
17. Hariz MI, Shamsgovara P, Johansson F, Hariz G, Fodstad H. Tolerance and tremor rebound following long-term chronic thalamic stimulation for Parkinsonian and essential tremor. Stereotact Funct Neurosurg. 1999. 72: 208-18
18. Holslag JAH, Neef N, Beudel M, Drost G, Oterdoom DLM, Kremer NI. Deep brain stimulation for essential tremor: A comparison of targets. World Neurosurg. 2018. 110: e580-4
19. Jankovic J, Tolosa E.editorsParkinson's Disease and Movement Disorders. Baltimore, MD: Williams and Wilkins; 1993. p.
20. Kitagawa M, Murata J, Kikuchi S, Sawamura Y, Saito H, Sasaki H. Deep brain stimulation of subthalamic area for severe proximal tremor. Neurology. 2000. 55: 114-6
21. Kocabicak E, Temel Y. Deep brain stimulation of the subthalamic nucleus in Parkinson's disease: Surgical technique, tips, tricks and complications. Clin Neurol Neurosurg. 2013. 115: 2318-23
22. Louis ED. Clinical practice. English J. 2001. 345: 1113-8
23. Louis ED. De Sedibus et Causis Morborum: Is essential tremor a primary disease of the cerebellum?. Cerebellum. 2016. 15: 233-4
24. Louis ED, Ferreira JJ. How common is the most common adult movement disorder? Update on the worldwide prevalence of essential tremor. Mov Disord. 2010. 25: 534-41
25. Lozano AM, Levy R. Reoperation of deep brain stimulation in patients with essential tremor. World Neurosurg. 2012. 78: 442-4
26. Miocinovic S, Somayajula S, Chitnis S, Vitek JL. History, applications, and mechanisms of deep brain stimulation. JAMA Neurol. 2013. 70: 163-71
27. Murata J, Kitagawa M, Uesugi H, Saito H, Iwasaki Y, Kikuchi S. Electrical stimulation of the posterior subthalamic area for the treatment of intractable proximal tremor. J Neurosurg. 2003. 99: 708-15
28. Pahwa R, Lyons KL, Wilkinson SB, Carpenter MA, Tröster AI, Searl JP. Bilateral thalamic stimulation for the treatment of essential tremor. Neurology. 1999. 53: 1447-50
29. Plaha P, Javed S, Agombar D, O’ Farrell G, Khan S, Whone A. Bilateral caudal zona incerta nucleus stimulation for essential tremor: Outcome and quality of life. J Neurol Neurosurg Psychiatry. 2011. 82: 899-904
30. Plaha P, Khan S, Gill SS. Bilateral stimulation of the caudal zona incerta nucleus for tremor control. J Neurol Neurosurg Psychiatry. 2008. 79: 504-13
31. Plaha P, Patel NK, Gill SS. Stimulation of the subthalamic region for essential tremor. J Neurosurg. 2004. 101: 48-54
32. Sandvik U, Koskinen LO, Lundquist A, Blomstedt P. Thalamic and subthalamic deep brain stimulation for essential tremor: Where is the optimal target?. Neurosurgery. 2012. 70: 840-5
33. Shih LC, LaFaver K, Lim C, Papavassiliou E, Tarsy D. Loss of benefit in VIM thalamic deep brain stimulation (DBS) for essential tremor (ET): How prevalent is it?. Park Relat Disord. 2013. 19: 676-9
34. Taha JM, Janszen MA, Favre J. Thalamic deep brain stimulation for the treatment of head, voice, and bilateral limb tremor. J Neurosurg. 1999. 91: 68-72
35. Tröster AI, Pahwa R, Fields JA, Tanner CM, Lyons KE. Quality of life in Essential Tremor Questionnaire (QUEST): Development and initial validation. Park Relat Disord. 2005. 11: 367-73