- Department of Surgical Specialties, Neurosurgery Teaching and Assistance Unit, Pedro Ernesto University Hospital, RJ, Brazil
- Department of Physiological Sciences, Roberto Alcântara Gomes Biology Institute, Rio de Janeiro State University, Rio de Janeiro, RJ, Brazil.
- Nervous System Electric Stimulation Laboratory (LabEEL), Neurosurgery Teaching and Assistance Unit, Pedro Ernesto University Hospital, RJ, Brazil
Correspondence Address:
Pedro Henrique da Costa Ferreira Pinto
Department of Surgical Specialties, Neurosurgery Teaching and Assistance Unit, Pedro Ernesto University Hospital, RJ, Brazil
DOI:10.25259/SNI-124-2019
Copyright: © 2019 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Pedro Henrique da Costa Ferreira Pinto, Flavio Nigri, Egas Moniz Caparelli-Dáquer, Jucilana dos Santos Viana. Computed tomography-guided navigated transcranial magnetic stimulation for preoperative brain motor mapping in brain lesion resection: A case report. 05-Jul-2019;10:134
How to cite this URL: Pedro Henrique da Costa Ferreira Pinto, Flavio Nigri, Egas Moniz Caparelli-Dáquer, Jucilana dos Santos Viana. Computed tomography-guided navigated transcranial magnetic stimulation for preoperative brain motor mapping in brain lesion resection: A case report. 05-Jul-2019;10:134. Available from: http://surgicalneurologyint.com/surgicalint-articles/9461/
Abstract
Background: Navigated transcranial magnetic stimulation (nTMS) is a well establish a noninvasive method for preoperative brain motor mapping. We commonly use magnetic resonance imaging (MRI) to supply the nTMS system. In some cases, MRI is not possible or available, and the use of computed tomography (CT) is necessary. We present the first report describing the association of CT and nTMS motor mapping for brain lesion resection.
Case Description: CT imaging of a 59-year-old man suffering from acquired immune deficiency syndrome for 17 years, presenting with seizure and right hemiparesis, revealed a small single hypodense ring-enhancing lesion in the left central sulci suggesting cerebral toxoplasmosis. After 3 weeks of neurotoxoplasmosis treatment, due to four consecutive tonic-clonic seizures, a new CT scan was performed and showed no lesion changes. MRI was in maintenance at that time. Infectious diseases department suggested a brain lesion biopsy. Due to lesion’s location, we decided to perform a presurgical nTMS motor mapping. After a small craniotomy, we could precisely locate and safely totally remove the lesion. The pathology report revealed a high suspicious toxoplasmosis pattern. The patient was discharged after 2 days and continued toxoplasmosis treatment. After 6 months follow-up, he showed no signs of any procedure-related deficits or radiological recurrence.
Conclusion: We report the feasibility and applicability of nTMS motor mapping using CT scan as an image source. It gives neurosurgeons another possibility to perform motor mapping for brain lesion removal, especially when MRI is not available or feasible.
Keywords: Brain biopsy, Brain lesion, Brain tumor, Computed tomography, Motor mapping, Navigated transcranial magnetic stimulation
INTRODUCTION
The imaging modality of choice in patients with brain lesions is magnetic resonance imaging (MRI).[
Since Barker et al.[
We present the first report describing the association of CT and nTMS motor mapping for brain lesion removal. This report shows the feasibility and usefulness of this method, enlarging possibilities in the neurosurgical armamentarium.
CLINICAL PRESENTATION
A 59-year-old man, previously diagnosed with acquired immune deficiency syndrome 17 years ago, had been treated at the infectious diseases department under antiretroviral therapy, using lamivudine, zidovudine, and efavirenz. He was admitted to our hospital with generalized tonic-clonic seizure and progressive right hemiparesis. Cranial CT scan (Phillips Diamond Select Brilliance 64-slice, slice thickness 1 mm, and slice interval 0.5 mm, Phillips, Eindhoven, Netherlands) revealed a single 8.8 mm × 10.5 mm × 9.2 mm ring-enhancing hypodense lesion in the left central sulci suggesting neurotoxoplasmosis. Cerebral toxoplasmosis therapy with sulfadiazine, pyrimethamine, and folinic acid was started and combined with lamotrigine for seizures. A good neurological recovery was observed, but after 3 weeks, he had four consecutive seizure episodes in a 3-h interval without impairment of motor function. A new cranial contrast CT scan showed the same lesion without improvement despite treatment. After discussion with infectious diseases staff, we decided to perform a diagnostic brain biopsy to guide treatment.
Due to the lesion’s location, we decided to do a presurgical mapping of the motor area using nTMS system. This includes a TMS (Rapid2, Magstim Corporation, Whitland, Wales, United Kingdom) combined with a navigation system (Brainsight 2.4, Rogue Research, Montreal, Canada) and tracking system (Polaris, Northern Digital Instruments, Waterloo, Canada). We depict stimulation sites on reconstructed brain surface CT images. Our nTMS routine consists of a patient seated comfortably in a chair with headrest [
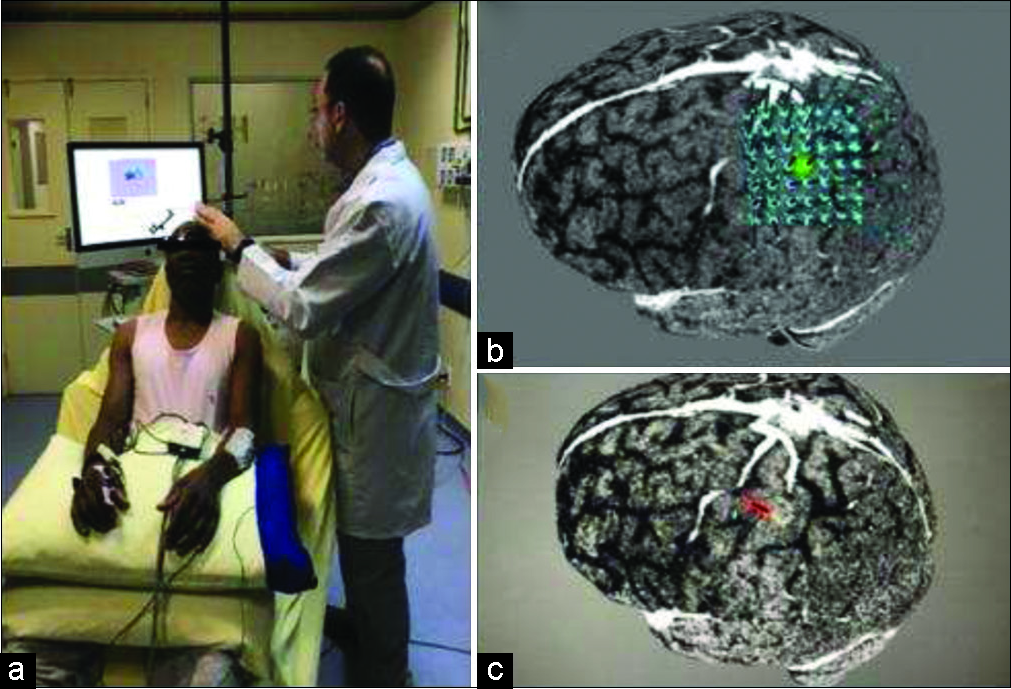
Figure 1:
Navigated transcranial magnetic stimulation motor mapping session. (a) Patient in a relaxing condition, with eyes open and superior limbs supported by pillows. (b) We centered a 5 × 5 cm2 grid on the lesion. (c) Result was a very close relation between anterior aspect of the lesion and the hand region of the primary motor cortex.
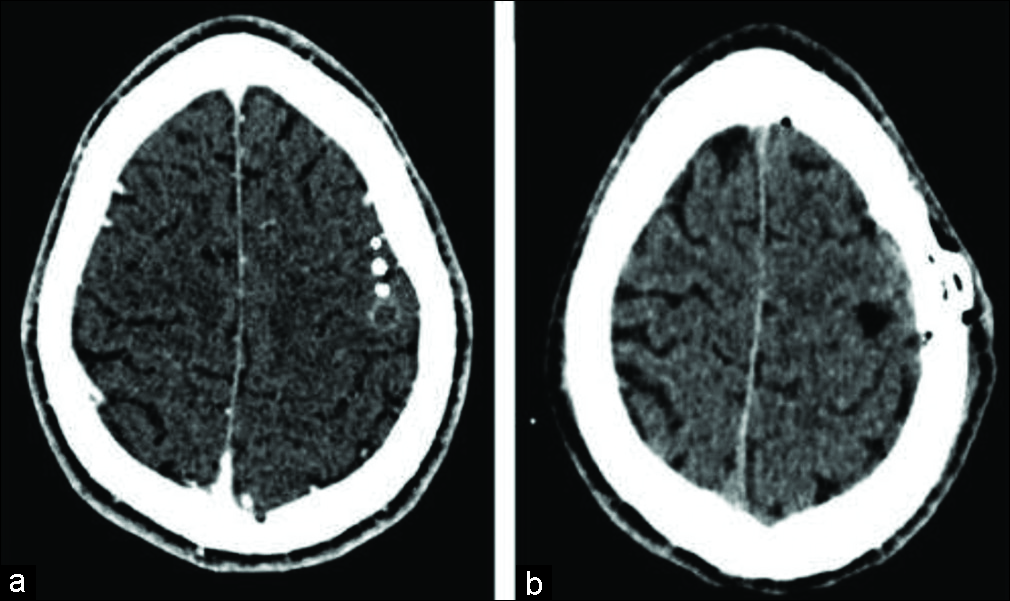
Figure 2:
Navigated transcranial magnetic stimulation motor mapping final result. Any motor evoked potential amplitude >50 microvolts (peak-to-peak) were considered positive and exported to digital imaging and communications in medicine format as a white spot. (a) Axial head computed tomography showing the white spots near from lesion area. (b) Sagittal head computed tomography showing the relationship of motor sites and lesion. Again, we can observe close proximity.
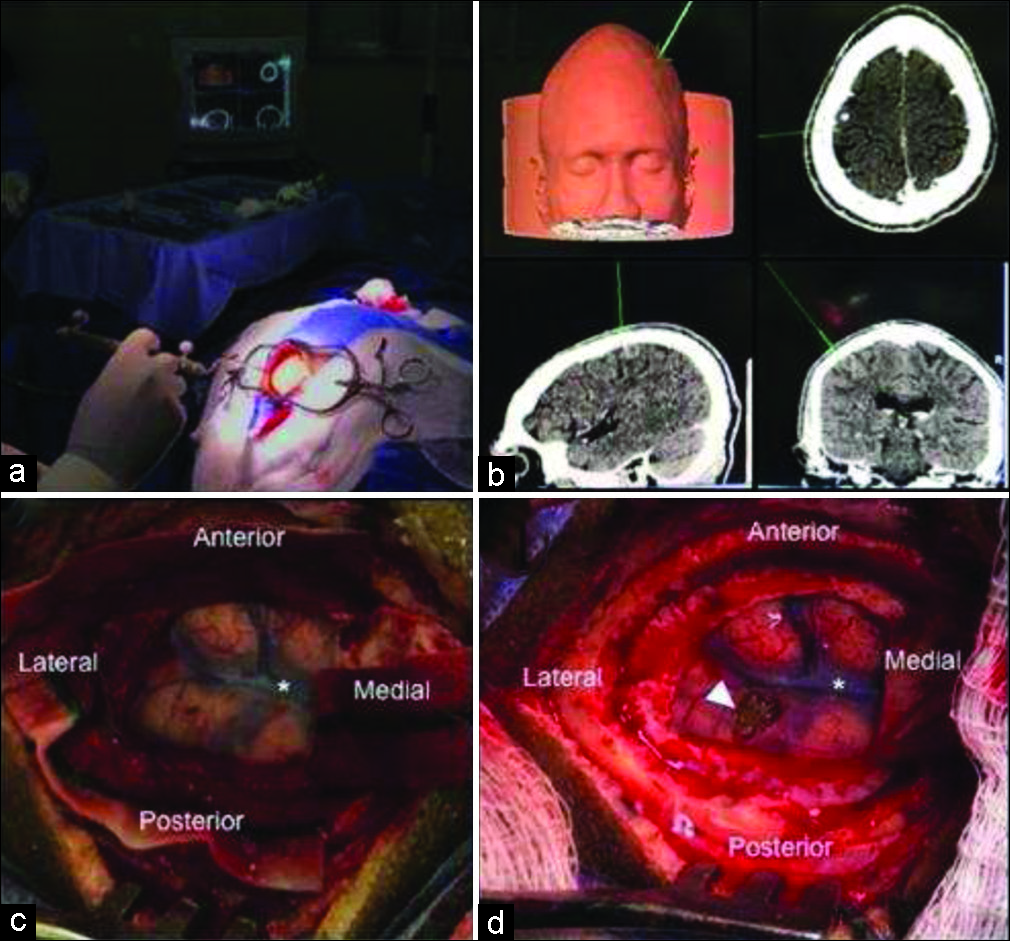
Figure 3:
Surgical steps of open brain lesion removal using computed tomography-guided navigated transcranial magnetic stimulation. (a) Localizing craniotomy site guided by neuronavigation system. (b) Neuronavigation system image could precisely localize lesion. (c) After durotomy, we exposed the eloquent cortex and lesion site. Asterisk represents a cortical vein on central sulcus between motor and somatosensory cortex. (d) Final aspect after complete lesion removal as seen in arrowhead.
DISCUSSION
The use of CT instead of MRI was necessary in this case because at that time the MRI was unavailable under maintenance and we needed to perform brain biopsy as soon as possible. The patient had four consecutive seizure episodes despite apparently successful initial treatment. Zoubi et al.[
Intraoperative cortical stimulation mapping is the gold standard method, but nTMS has a very good correlation of motor points and can be performed before surgery, allowing presurgical planning.[
CONCLUSION
We report the feasibility and applicability of nTMS motor mapping using CT scan as an image source. This paper does not have the intention to substitute MRI for CT scan in patients with brain lesions. It just gives another possibility to perform motor mapping for brain surgery, especially when MRI is not available or feasible. There is little information about CT guided nTMS in literature and therefore validation through further studies on the subject is necessary.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patient has given her consent for her images and other clinical information to be reported in the journal. The patient understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Authors received financial support from Rio de Janeiro State Research Support Foundation - Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ) and Center of High Complexity Neurosurgery Intern Patients - Núcleo de Internação de Pacientes Neurocirúrgicos de Alta Complexidade (NIPNAC).
Conflicts of interest
There are no conflicts of interest.
References
1. Barker AT, Jalinous R, Freeston IL. Non-invasive magnetic stimulation of human motor cortex. Lancet. 1985. 1: 1106-7
2. Bartek J, Cooray G, Islam M, Jensdottir M.editors. Stereotactic Brain Biopsy in Eloquent Areas Assisted by Navigated Transcranial Magnetic Stimulation. A Technical Case Report. Oper Neurosurg (Hagerstown). p.
3. Bradley WG, Waluch V, Yadley RA, Wycoff RR. Comparison of CT and MR in 400 patients with suspected disease of the brain and cervical spinal cord. Radiology. 1984. 152: 695-702
4. Brasil-Neto JP, Cohen LG, Panizza M, Nilsson J, Roth BJ, Hallett M. Optimal focal transcranial magnetic activation of the human motor cortex: Effects of coil orientation, shape of the induced current pulse, and stimulus intensity. J Clin Neurophysiol. 1992. 9: 132-6
5. Brighina F, Curatolo M, Cosentino G, De Tommaso M, Battaglia G, Sarzi-Puttini PC. Brain modulation by electric currents in fibromyalgia: A Structured review on non-invasive approach with transcranial electrical stimulation. Front Hum Neurosci. 2019. 13: 40-
6. Dell’osso B, D’Urso N, Castellano F, Ciabatti M, Altamura AC. Long-term efficacy after acute augmentative repetitive transcranial magnetic stimulation in bipolar depression: A 1-year follow-up study. J ECT. 2011. 27: 141-4
7. Di Lazzaro V, Oliviero A, Mazzone P, Insola A, Pilato F, Saturno E. Comparison of descending volleys evoked by monophasic and biphasic magnetic stimulation of the motor cortex in conscious humans. Exp Brain Res. 2001. 141: 121-7
8. Forster MT, Hattingen E, Senft C, Gasser T, Seifert V, Szelényi A. Navigated transcranial magnetic stimulation and functional magnetic resonance imaging: Advanced adjuncts in preoperative planning for central region tumors. Neurosurgery. 2011. 68: 1317-24
9. Jacobs AH, Kracht LW, Gossmann A, Rüger MA, Thomas AV, Thiel A. Imaging in neurooncology. NeuroRx. 2005. 2: 333-47
10. Kammer T, Beck S, Thielscher A, Laubis-Herrmann U, Topka H. Motor thresholds in humans: A transcranial magnetic stimulation study comparing different pulse waveforms, current directions and stimulator types. Clin Neurophysiol. 2001. 112: 250-8
11. Krieg SM, Lioumis P, Mäkelä JP, Wilenius J, Karhu J, Hannula H. Protocol for motor and language mapping by navigated TMS in patients and healthy volunteers; workshop report. Acta Neurochir (Wien). 2017. 159: 1187-95
12. Krieg SM, Shiban E, Buchmann N, Gempt J, Foerschler A, Meyer B. Utility of presurgical navigated transcranial magnetic brain stimulation for the resection of tumors in eloquent motor areas. J Neurosurg. 2012. 116: 994-1001
13. Krings T, Foltys H, Reinges MH, Kemeny S, Rohde V, Spetzger U. Navigated transcranial magnetic stimulation for presurgical planning correlation with functional MRI. Minim Invasive Neurosurg. 2001. 44: 234-9
14. Lawson McLean A, Frank S, Zafar N, Waschke A, Kalff R, Reichart R. Time course of the response to navigated repetitive transcranial magnetic stimulation at 10 Hz in chronic neuropathic pain. Neurol Res. 2018. 40: 564-72
15. Perkins A, Liu G. Primary brain tumors in adults: Diagnosis and treatment. Am Fam Physician. 2016. 93: 211-7
16. Picht T, Mularski S, Kuehn B, Vajkoczy P, Kombos T, Suess O. Navigated transcranial magnetic stimulation for preoperative functional diagnostics in brain neurosurgery. Neurosurgery. 2009. 65: 93-8
17. Picht T, Schmidt S, Brandt S, Frey D, Hannula H, Neuvonen T. Preoperative functional mapping for rolandic brain tumor surgery: Comparison of navigated transcranial magnetic stimulation to direct cortical stimulation. Neurosurgery. 2011. 69: 581-8
18. Rossini PM, Burke D, Chen R, Cohen LG, Daskalakis Z, Di Iorio R. Non-invasive electrical and magnetic stimulation of the brain, spinal cord, roots and peripheral nerves: Basic principles and procedures for routine clinical and research application. An updated report from an I.F.C.N. Committee. Clin Neurophysiol. 2015. 126: 1071-107
19. Takakura T, Muragaki Y, Tamura M, Maruyama T, Nitta M, Niki C. Navigated transcranial magnetic stimulation for glioma removal: Prognostic value in motor function recovery from postsurgical neurological deficits. J Neurosurg. 2017. 127: 877-91
20. Tarapore PE, Tate MC, Findlay AM, Honma SM, Mizuiri D, Berger MS. Preoperative multimodal motor mapping: A comparison of magnetoencephalography imaging, navigated transcranial magnetic stimulation, and direct cortical stimulation. J Neurosurg. 2012. 117: 354-62
21. Yabuuchi H, Kamitani T, Sagiyama K, Yamasaki Y, Matsuura Y, Hino T. Clinical application of radiation dose reduction for head and neck CT. Eur J Radiol. 2018. 107: 209-15
22. Zhou L, Guo Z, Xing G, Peng H, Cai M, Chen H. Antidepressant effects of repetitive transcranial magnetic stimulation over prefrontal cortex of parkinson’s disease patients with depression: A meta-analysis. Front Psychiatry. 2019. 9: 769-
23. Zoubi MA, Zulfiqar B, Kulkarni M. Cerebral toxoplasmosis requiring urgent brain biopsy. IDCases. 2017. 9: 59-61