- IPSPAC – Instituto Paulista de Saúde para a Alta Complexidade. 6, Santo André, São Paulo, Brazil
- Division of Neurosurgery, Hospital das Clínicas, Faculdade de Medicina da Universidade de São Paulo, São Paulo, Brazil
Correspondence Address:
Sabrina A. de França
Division of Neurosurgery, Hospital das Clínicas, Faculdade de Medicina da Universidade de São Paulo, São Paulo, Brazil
DOI:10.4103/sni.sni_388_17
Copyright: © 2018 Surgical Neurology International This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.How to cite this article: Sabrina A. de França, Thales B. Nepomuceno, Wellingson S. Paiva, Almir F. Andrade, Manoel J. Teixeira, Wagner M. Tavares. Cranial autologous bone flap resorption after a cranioplasty: A case report. 19-Mar-2018;9:61
How to cite this URL: Sabrina A. de França, Thales B. Nepomuceno, Wellingson S. Paiva, Almir F. Andrade, Manoel J. Teixeira, Wagner M. Tavares. Cranial autologous bone flap resorption after a cranioplasty: A case report. 19-Mar-2018;9:61. Available from: http://surgicalneurologyint.com/surgicalint-articles/cranial-autologous-bone-flap-resorption-after-a-cranioplasty-a-case-report/
Abstract
Background:Craniectomies and cranioplasty are common neurosurgical procedures performed after brain trauma, ischemia, tumor resection, or infection. Post-cranioplasty autologous bone flap resorption may occur in patients after delayed cranial reconstruction. The occurrence is usually low when bone flaps are stored in subcutaneous abdominal tissue. We report a unique case of post-cranioplasty cranial bone flap.
Case Description:We report a total autologous bone flap resorption in a 28-year-old man with a history of alcohol abuse. He was found unconscious in his bedroom with a head trauma of unknown mechanism. After an emergency room assessment, he was diagnosed with an acute subdural hematoma and underwent to emergency surgical drainage and a craniectomy. Three months later, a cranioplasty was performed and he exhibited exceptional outcomes. During a follow-up assessment, 7 months post-cranioplasty, total bone flap resorption was observed on computerized tomography image.
Conclusion:This case described an abnormal accelerated resorption of an autologous bone flap cranioplasty inserted after 3 months. Thus, to avoid bone flap resorption, an as early as possibly strategy may prevent this. Still, the exact mechanisms underlying bone resorption are poorly understood.
Keywords: Autologous bone flap, cranioplasty, decompressive craniectomy, resorption
INTRODUCTION
Cranioplasty is the surgical reconstruction of cranial bone defects. It frequently follows the treatment of conditions, such as multifragmentary fractures, decompressive craniectomies, tumors, and infections of a previous surgical site.[
Various materials can be applied, including polyethylene, acrylic resins, titanium, and autologous bone grafts. Bone grafts can be sourced from ribs, iliac bones or, for optimal cosmetic outcomes, explanted calvarial bones.[
In the case of autologous calvarial bone, it is important to preserve the bone flap for future re-implantation (cranial reconstruction), and there are three storage options:[
Here, we report a case of an unusual accelerated autologous bone flap resorption after cranial reconstruction
CASE REPORT
A 28-year-old man, with history of chronic alcohol abuse was found unconscious, with approximately 24 h after a traumatic brain injury (TBI) of unknown mechanism. He was admitted to our hospital for neurologic assessment and management. As recommended by the American College of Surgeons,[
He was immediately transferred to the operating room and, after balanced standard general anesthesia, he received a left question mark incision and a fronto-temporo-parietal craniectomy. After a durotomy and acute subdural drainage, he underwent dura mater reconstruction using galea aponeurotica. After standard subcutaneous tissue and skin closures, the extracted bone flap was bisected via osteotomy and both parts were subcutaneously stored in his left abdomen.
Immediately after the procedure, the patient underwent postoperative CT scanning before his intensive care unit (ICU) admission. This revealed an acute epidural hematoma, so he was returned to operating room for further neurosurgery. After standard balanced general anesthesia, the question mark incision was reopened and the hematoma was removed. No active bleeding site was identified. After careful hemostasis, the skin flap was sutured shut, and the patient was transferred to the ICU.
Because the patient presented diffuse brain edema, he was postoperatively sedated for 2 days, at which point CT scanning confirmed that his condition had improved. However, he was mildly sedated during the time of extubation. He developed pneumonia due mechanical ventilation, so he was tracheostomized and discharged from ICU without mechanical ventilation. Seven days before his hospital discharge, his tracheostomy cannula was withdrawn.
The patient underwent repeated neurological examinations in the ICU. His pupils were anisocoric throughout his hospitalization. His Glasgow Scale score improved to 10T in the ICU and progressed until 15 after his ICU discharge. No motor deficits were identified at discharge.
At his hospital discharge, which happened 33 days after the first procedure, he was orientated and alert but still had a left third cranial nerve deficit.
Cranioplasty and follow-up
One hundred and four days after craniectomy, the patient underwent a cranioplasty with his two abdominally stored autologous bone flaps. His head was positioned in a horseshoe-shaped restraint and the question mark incision was antiseptically reopened. A dissection was made between the galea and the dura mater to isolate the craniectomy's border. The abdominal incision was reopened to withdraw the bone flaps.
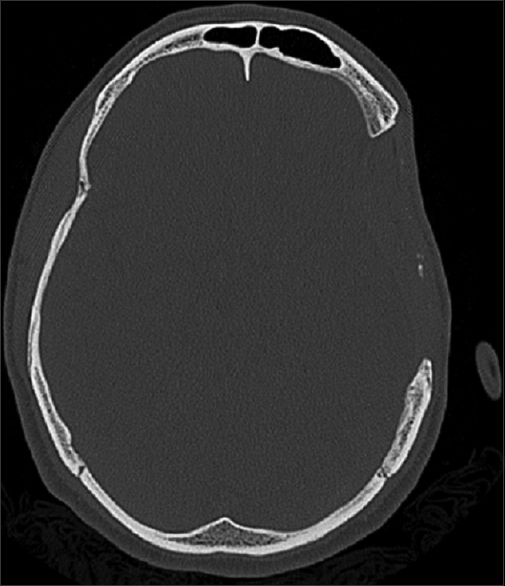
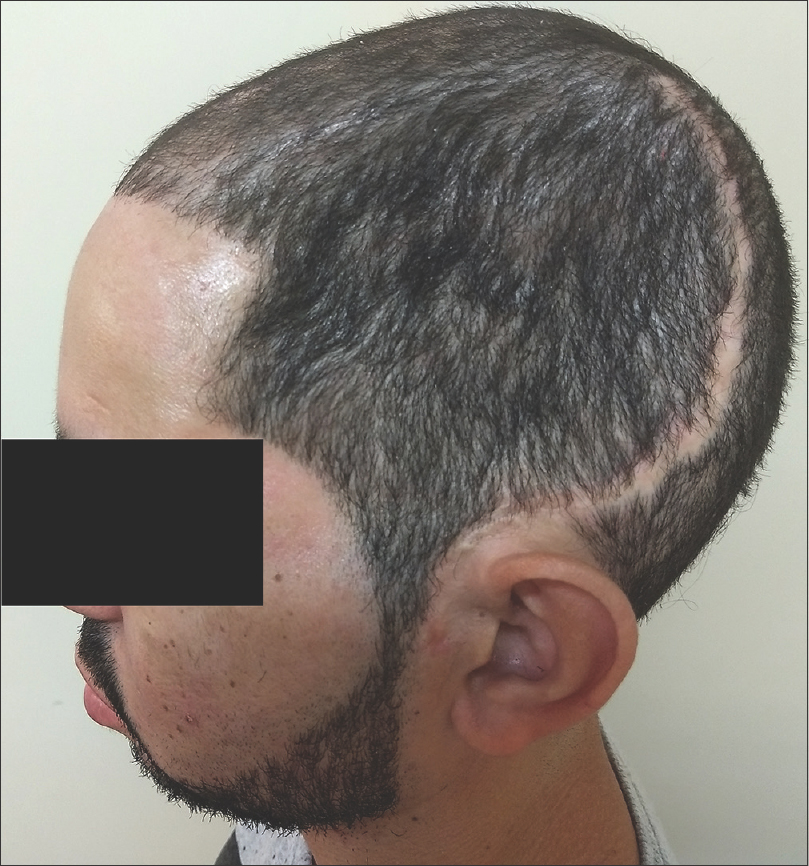
During evaluation, signs of bone resorption were detected at the bone flaps temporal parts, but no corrections were made due to the poor esthetic results typically obtained from acrylic mold.
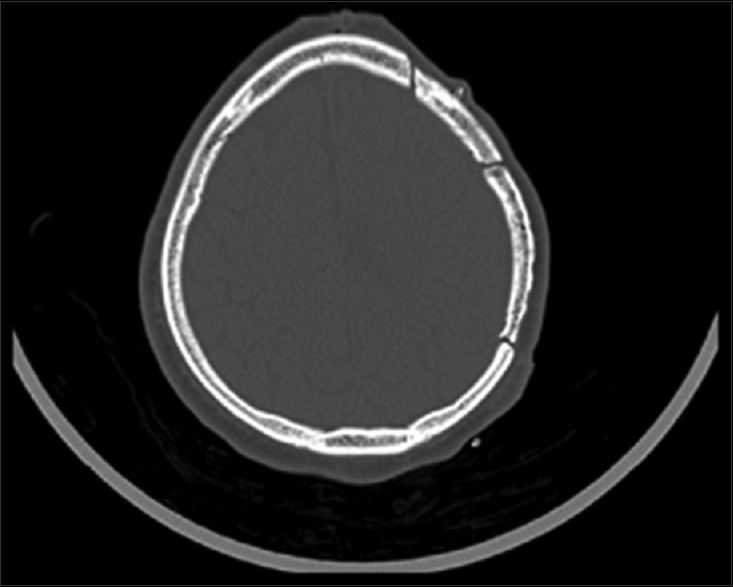
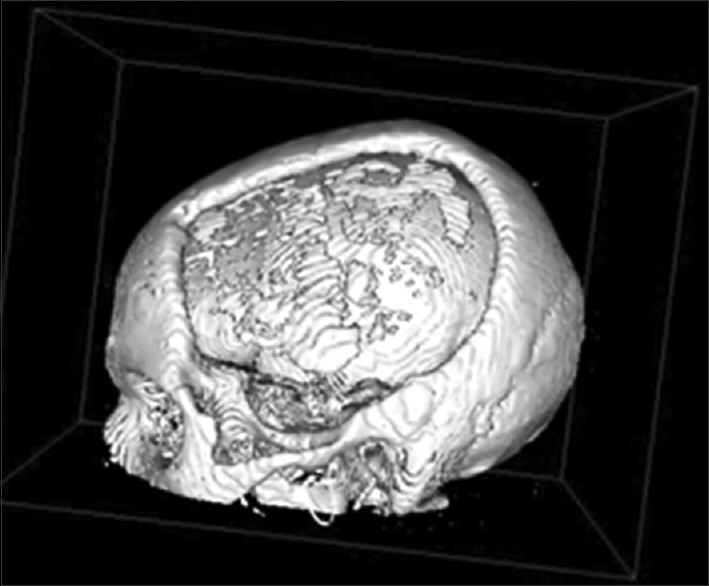
Ten months after the craniectomy and 7 months after the cranioplasty, the patient returned to the trauma clinic with secondary bone defects related to near complete. It reached almost 95% of bone flap resorption, as proven in CT scan in Figures
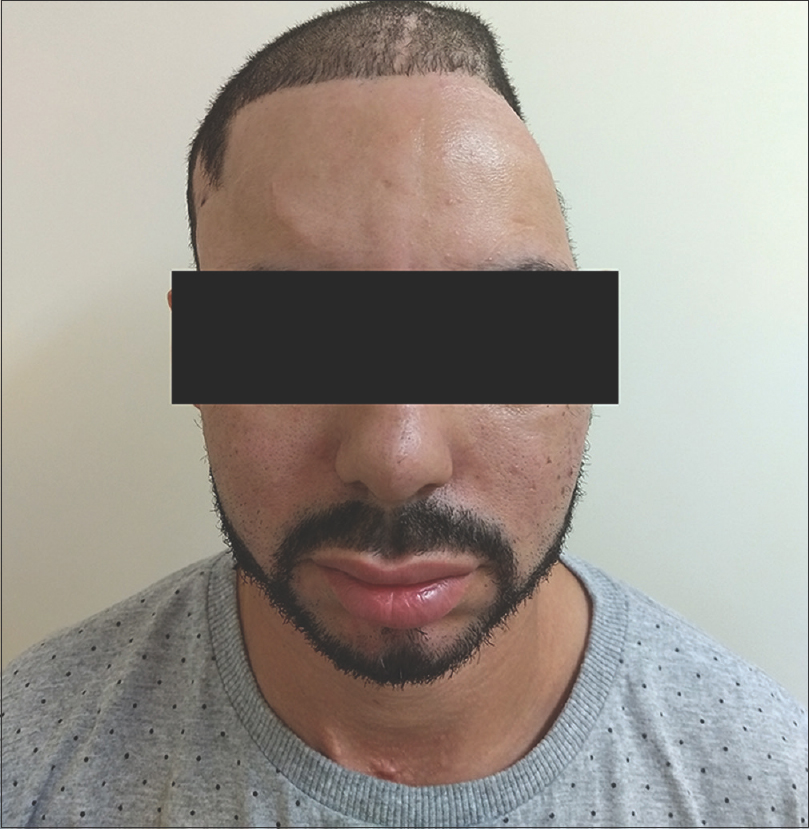
The patient is waiting for secondary cranioplasty with allogenic material and has made a seemly good neurological recovery considering the primary situation he had score 6 in Glasgow Outcome Scale – Extended.
DISCUSSION
One of the earliest and most remarkable descriptions of successful bone flap storage in the abdomen was published in 1920 by Kreinder.[
Bone flap viability depends on several complex processes. Revascularization, osteogenesis, osteoinduction, and osteoconduction must be balanced. Revascularization applies to the capillaries from the surrounding bone, dura, and scalp that enter the bone flap, induce proliferation of granular tissue, and initiate osteoinduction,[
Movassaghi et al.[
Certain bone flap treatment methods, such as irradiation, and deep-freezing, can denature proteins involved in osteoinduction and osteoconduction, and thus promote bone resorption.[
Furthermore, several studies[
Bone flap resorption is very common complication after cranioplasty in children.[
CONCLUSION
This case described an abnormal accelerated resorption of an autologous bone flap cranioplasty inserted after 3 months. There is probably a window of opportunity for bone re-implantation before the proteins involved in osteoinduction and osteoconduction degrade. Still, the exact mechanisms underlying bone resorption are poorly understood and whether an earlier cranioplasty would have avoided the resorption is unknown. Still, our case shows that the time window for re-implantation may not be as long as previously believed or at least that the time-window is probably not the same for every patient.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Albrektsson T, Johansson C. Osteoinduction, osteoconduction and osseointegration. Eur Spine J. 2001. 10: S96-S101
2. Asano Y, Ryuke Y, Hasuo M, Simosawa S. Cranioplasty using cryopreserved autogenous bone [Japanese]. No To Shinkei. 1993. 45: 1145-50
3. Brommeland T, Rydning PN, Pripp AH, Helseth E. Cranioplasty complications and risk factors associated with bone flap resorption. Scand J Trauma Resusc Emerg Med. 2015. 23: 75-
4. Chapleau W, Al-Khatib J, Haskin D. Advanced trauma life support (ATLS®): The ninth edition. J Trauma Acute Care Surg. 2013. 74: 1363-6
5. Dünisch P, Walter J, Sakr Y, Kalff R, Waschke A, Ewald C. Risk factors of aseptic bone resorption: A study after autologous bone flap reinsertion due to decompressive craniotomy. J Neurosurg. 2013. 118: 1141-7
6. Fernandes Joaquim A, Paulo Mattos J, Chaddad Neto F, Lopes A, de Oliveira E. Bone flap management in neurosurgery. Revista Neurociências. 2009. 17: 133-7
7. Flannery T, McConnell RS. Cranioplasty: Why throw the bone flap out?. Br J Neurosurg. 2001. 15: 518-20
8. Gilbert SF.editors. Osteogenesis: The Development of Bones. Sunderland, MA: Sinauer Associates; 2000. p.
9. Gooch MR, Gin GE, Kenning TJ, German JW. Complications of cranioplasty following decompressive craniectomy: Analysis of 62 cases. Neurosurg Focus. 2009. 26: E9-
10. Grossman N, Shemesh-Jan HS, Merkin V, Gideon M, Cohen A. Deep-freeze preservation of cranial bones for future cranioplasty: Nine years of experience in Soroka University Medical Center. Cell Tissue Bank. 2007. 8: 243-6
11. Häuptli J, Segantini P. New tissue preservation method for bone flaps following decompressive craniotomy [German]. Helv Chir Acta 1980;47:121-4Redfern RM, Pülhorn H. Cranioplasty. Adv Clin Neurosci Reabil. 2007. 7: 32-4
12. Hng D, Bhaskar I, Khan M, Budgeon C, Damodaran O, Knuckey N. Delayed Cranioplasty: Outcomes Using Frozen Autologous Bone Flaps. Craniomaxillofac Trauma Reconstr. 2015. 8: 190-7
13. Josan VA, Sgouros S, Walsh AR, Dover MS, Nishikawa H, Hockley AD. Cranioplasty in children. Childs Nerv Syst. 2005. 21: 200-4
14. Kim JS, Cheong JH, Ryu JI, Kim JM, Kim CH. Bone flap resorption following cranioplasty after decompressive craniectomy: Preliminary report. Korean J Neurotrauma. 2015. 11: 1-5
15. Kreider GN. Repair of cranial defect by new method. JAMA. 1920. 74: 1024-
16. Martin KD, Franz B, Kirsch M, Polanski W, von der Hagen M, Schackert G. Autologous bone flap cranioplasty following decompressive craniectomy is combined with a high complication rate in pediatric traumatic brain injury patients. Acta Neurochir (Wien). 2014. 156: 813-24
17. Morina A, Kelmendi F, Morina Q, Dragusha S, Ahmeti F, Morina D. Cranioplasty with subcutaneously preserved autologous bone grafts in abdominal wall – Experience with 75 cases in a post-war country Kosova. Surg Neurol Int. 2011. 2: 72-
18. Movassaghi K, Ver Halen J, Ganchi P, Amin-Hanjani S, Mesa J, Yaremchuk MJ. Cranioplasty with subcutaneously preserved autologous bone grafts. Plast Reconstr Surg. 2006. 117: 202-6
19. Osawa M, Hara H, Ichinose Y, Koyama T, Kobayashi S, Sugita Y. Cranioplasty with a frozen and autoclaved bone flap. Acta Neurochir. 1990. 102: 38-41
20. Redfern RM, Pülhorn H. Cranioplasty. Adv Clin Neurosci Reabil. 2007. 7: 32-4
21. Rocque BG, Amancherla K, Lew SM, Lam S. Outcomes of cranioplasty following decompressive craniectomy in the pediatric population. J Neurosurg Pediatr. 2013. 12: 120-5
22. Shoakazemi A, Flannery T, McConnell RS. Long-term outcome of subcutaneously preserved autologous cranioplasty. Neurosurgery. 2009. 65: 505-10
23. Staffa G, Nataloni A, Compagnone C, Servadei F. Custom made cranioplasty prostheses in porous hydroxy-apatite using 3D design techniques: 7 years experience in 25 patients. Acta Neurochir. 2007. 149: 161-70
24. Sundseth J, Sundseth A, Berg-Johnsen J, Sorteberg W, Lindegaard KF. Cranioplasty with autologous cryopreserved bone after decompressive craniectomy: Complications and risk factors for developing surgical site infection. Acta Neurochir. 2014. 156: 805-11
25. Tybor K, Fortuniak J, Komuński P, Papiez T, Andrzejak S, Jaskólski D. Supplementation of cranial defects by an autologous bone flap stored in the abdominal wall [Polish]. Neurol Neurochir Pol. 2005. 39: 220-4