- Department of Neurosurgery, AIIMS Patna, Bihar, India.
- Department of Neurosurgery, Max Superspeciality Hospital, New Delhi, India.
Correspondence Address:
Saraj Singh
Department of Neurosurgery, Max Superspeciality Hospital, New Delhi, India.
DOI:10.25259/SNI_29_2019
Copyright: © 2019 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Saraj Singh, Rakesh Singh, Kapil Jain, Bipin Walia. Cranioplasty following decompressive craniectomy – Analysis of complication rates and neurological outcomes: A single center study. 19-Jul-2019;10:142
How to cite this URL: Saraj Singh, Rakesh Singh, Kapil Jain, Bipin Walia. Cranioplasty following decompressive craniectomy – Analysis of complication rates and neurological outcomes: A single center study. 19-Jul-2019;10:142. Available from: http://surgicalneurologyint.com/surgicalint-articles/9516/
Abstract
Background: Cranioplasty is the surgical intervention to repair cranial defects in both cosmetic and functional ways. Despite the fact that cranioplasty is a simple procedure, it is still associated with a relatively high complication rate, ranging between series from 12% to 50%.
Methods: The author did a prospective cohort study of patients from August 2015 to December 2017, who had undergone decompressive craniectomy followed by cranioplasty after 6 weeks at our institution. All patients were followed up to 6 months after cranioplasty and complications were recorded both by imaging and clinically. The complications were classified as minor (subgaleal collection, seizures) who did not require the second surgery and major (hydrocephalus, bone flap infection) who required the second surgery. To find out neurological outcome, Glasgow coma score (GCS) and Glasgow outcome scale extended (GOSE) were recorded at 1 month, 3 months, and 6 months.
Results: Overall complication rate in this study was 22.4% (16/72). Subgaleal collection was the most common complication (5.6%), followed by hydrocephalus (4.2%), seizure (4.2%), bone flap infection (2.8%), intracerebral hematoma (2.8%), empyema (1.4%), and subdural hematoma (SDH) (1.4%). Of these, 8.4% (n = 6/72) were major complication (hydrocephalus n = 3, bone flap infection n = 2, and SDH n = 1) which required the second surgery. GCS and GOSE were assessed preoperatively and in postoperative period at 1 month, 3 months, and 6 months. Both mean values of GCS and GOSE showed a significant improvement at 3 and 6 months after cranioplasty.
Conclusion: Cranioplasty after decompressive craniectomy is associated with higher complication rate, but good neurological outcome after surgery always outweighs the complications.
Key Message: Cranioplasty after decompressive craniectomy is associated with higher complication rate, but good neurological outcome after surgery always outweighs the complications. However, complications rate can be brought down by meticulous timing of cranioplasty in a patient of well-controlled comorbidities and precise surgical techniques. However, storing bone in bone bank is not an additional factor for any postcranioplasty complications which was considered previously.
Keywords: Cranioplasty, Hydrocephalus, Infection, Seizure
INTRODUCTION
Cranioplasty is the surgical procedure for repairing cranial defects in both cosmetic and functional ways. The history of cranioplasty dates back to 7000 B.C.[
Despite being a simple, safe, and clean procedure, it is still associated with a high complication rate, ranging from 12% to 50%.[
Cranioplasty also helps to optimize neurological recovery, both physiologically and/or clinically in patients of decompressive craniectomy.[
By this prospective study, we aim to review the complications and neurological outcome of cranioplasty following decompressive craniectomy.
MATERIALS AND METHODS
Consecutive patients of decompressive craniectomy at our institution were considered for the study. The study was conducted from August 2015 to December 2017. We conducted a prospective observational cohort study of 72 patients of cranioplasty following decompressive craniectomy. For the purpose of calculation of sample, complication rate has been considered as primary variable. Allowing an error of 10% either side, the sample size came to 67 with confidence level 95%. The formula used for calculating the sample size was as follows:
n=Z2a/2 π (1-π)/L2
Where, π =0.116; L= 0.10; and za/2=1.96.
Inclusion criteria
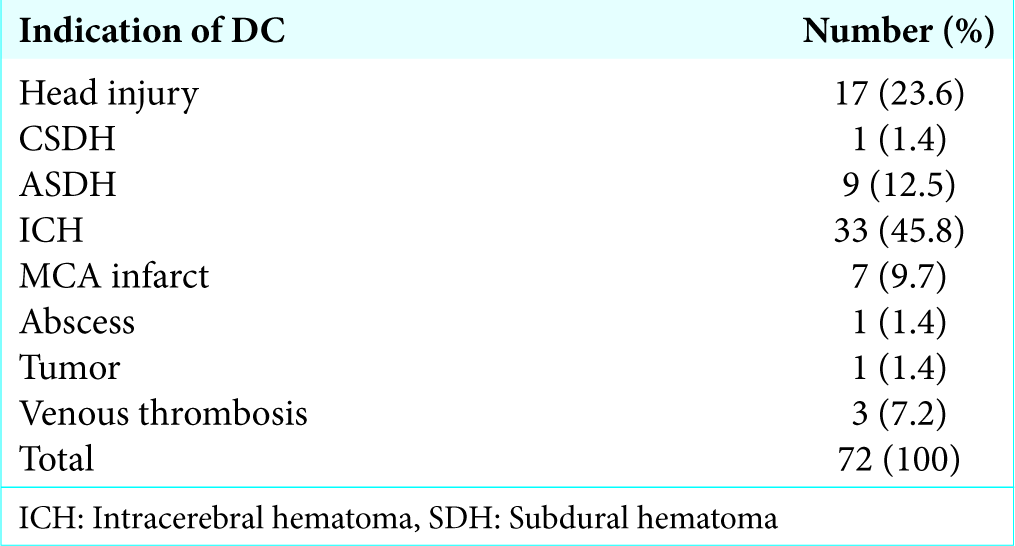
All adult patients above 18 years who underwent decompressive craniectomy for raised intracranial pressure due to various indications – nonpenetrating traumatic brain injury, intracerebral hematoma (ICH), subdural hematoma (SDH), and ischemic stroke, abscess, and tumor, were included in our study population.
Exclusion criteria
Patients not willing to participate in the study were excluded from the study.
Data collection methods
Data were collected prospectively when the patient was in individual participant data in the form of demographic profile at the time of cranioplasty (age, sex, indication of decompressive craniectomy, and laterality of decompressive craniectomy), comorbidity (hypertension, diabetes mellitus [DM], and use of antiplatelet drug), Glasgow coma score (GCS) and Glasgow outcome scale extended (GOSE) at the time of cranioplasty, intraoperative blood loss (ml), operative time (minute), and duration from decompressive craniectomy (days). All patients were followed up to 6 months of cranioplasty and complications were recorded. The complications were classified as minor who did not require the second surgery and major who required the second surgery. Postoperative computed tomography (CT) scan was done in all patients and findings were recorded. CT finding of the patient who developed complications was recorded separately. To find out neurological outcome, GCS and GOSE were recorded at 1 month, 3 months, and 6 months.
Methods of the measurement of outcome of interest
Primary outcome
To find out complications, all patients were followed at 1 month, 3 months, and 6 months and radiological assessment was done whenever required by CT scan.
Statistical analysis was done to find out relation of complication with gender, comorbidity (hypertension, DM, use of antiplatelet drug, and intraoperative events [blood loss and operative time]), and duration from decompressive craniectomy.
Secondary outcome
To review neurological outcome, all patients were followed and GCS and GOSE scale were recorded at time of admission, at 1 month, 3 months, and 6 months after cranioplasty.
Statistical analysis
Analysis was done by Student’s t-test, Chi-square tests, and Fisher’s exact tests and the results computed.
RESULTS
There were 74 patients who underwent cranioplasty during the study period, of which two were lost to follow-up after surgery. Remaining 72 patients were included in the study.
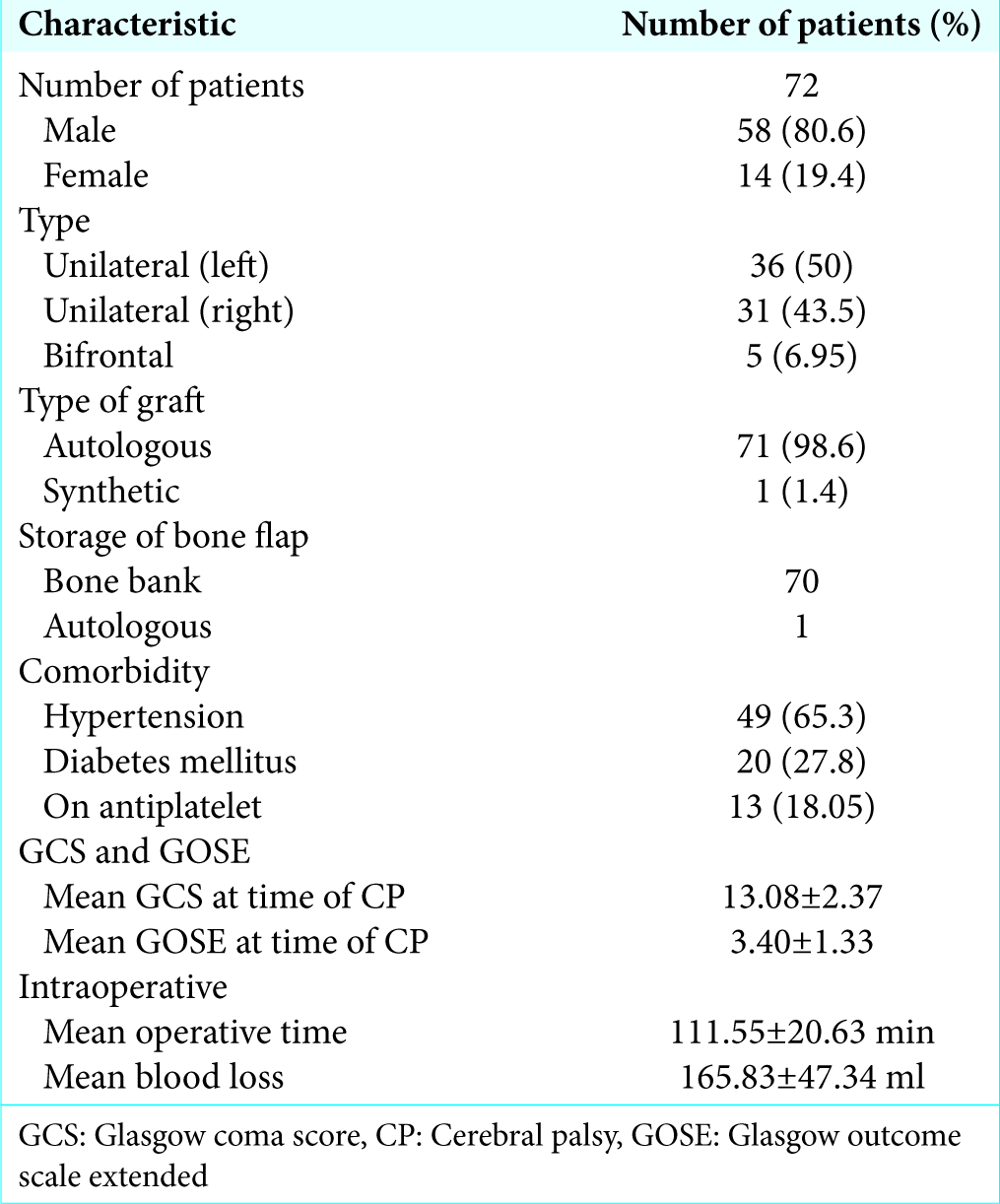
Of 72 patients, 58 were male (80.6%) and 14 were female. Mean age at the time of cranioplasty was 51.13 ± 16.96 (mean ± standard deviation) years. Majority (n = 67) had unilateral defect (93.05%) while 5 patients (6.95%) had bifrontal defect as a result of bifrontal decompressive craniectomy. The left side (n = 36) was more common (50%) than (n = 31) the right side (43.05%). Majority of patient had hypertension (65.3%) while 27.8% of patients were having DM. Thirteen patients (18.05%) were taking antiplatelets on regular basis [
Neurological outcome
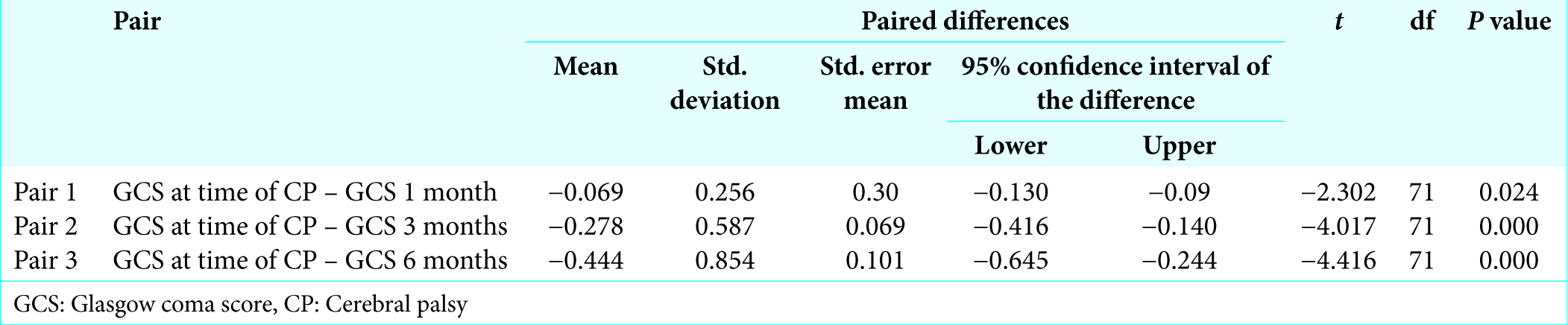
Patients had mean GCS of 13.08 ± 2.37at the time of cranioplasty, 13.15 ± 2.377 after 1 month of cranioplasty, 13.36 ± 2.241 after 3 months of cranioplasty, and 13.53 ± 2.22 6 months after cranioplasty. Statistical analysis was done to find out difference of GCS at 1 month, 3 months, and 6 months which showed that there was a significant difference (improvement) of GCS at 1 month, 3 months, and 6 months (P < 0.05) after cranioplasty [
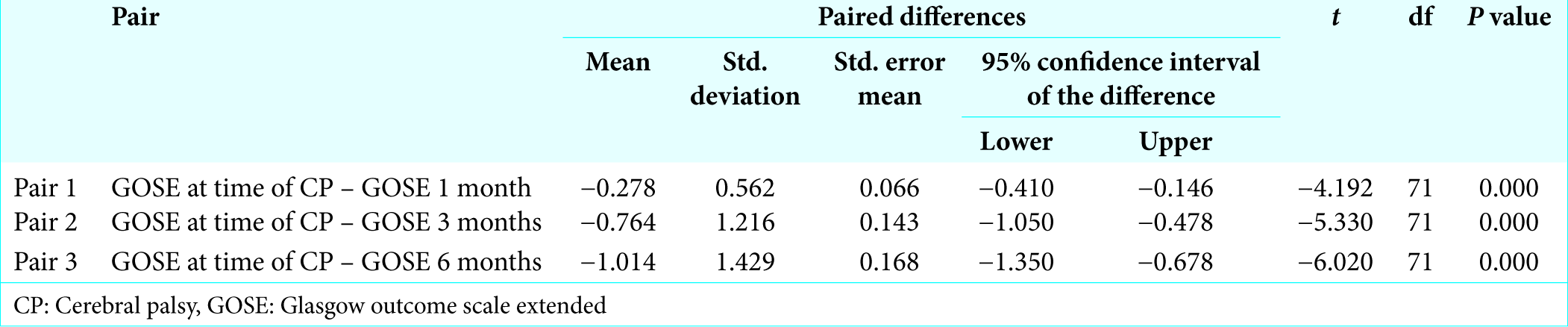
Patient had mean GOSE at time of cranioplasty 3.40 ± 1.34, 3.68 ± 1.432 after 1 month of cranioplasty, 4.17 ± 1.869 after 3 months of cranioplasty, and 4.42 ± 1.97 after 6 months of cranioplasty. Statistical analysis was done to find out difference of GOSE at 1 month, 3 months, and 6 months which showed that there was a significant difference (improvement) of GOSE at 1 month, 3 months, and 6 months (P < 0.05) after cranioplasty [
DISCUSSION
Cranioplasty is necessary for optimal neurological recovery after decompressive craniotomy. Anatomical reconstruction of skull after decompression always restructures the physiological balance of brain and its circulation. Along with technical aspects, emphasis on postoperative complications following cranioplasty should be given.[
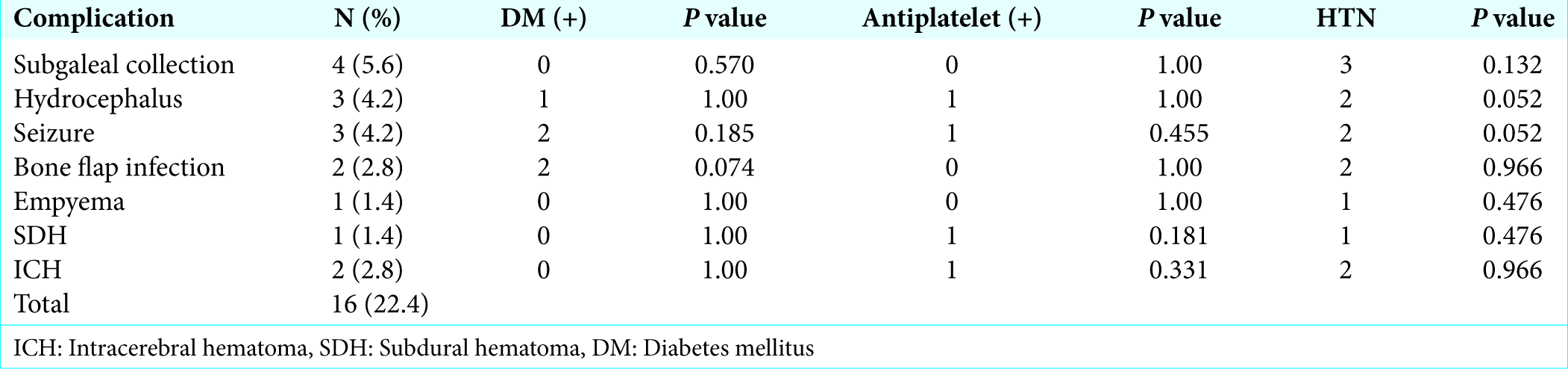
Overall complication rate in our study was 22.4% (16/72). Subgaleal collection was the most common (5.6%, n = 4) complication. All patients were managed conservatively. Repeated aspiration followed by crepe bandage application was done in three patients while one patient required lumber drain. Culture of aspirated fluid was sent in all cases which were sterile.
Two patients developed seizures (4.2%) (one patient had partial seizure and one patient had generalized tonic-clonic seizure). Both episodes occurred on the 1st postoperative day. CT head was done in both the patients, but there was no evidence of any contusion or hematoma. Antiepileptic drugs were modified in all patients and there were no further episodes till the last follow-up.
Two patients developed intracerebral hematoma which was detected on routine postoperative CT head. The size of hematoma was small and both patients did not develop new neurological deficit so they were managed conservatively.
One patient (1.4%) developed empyema on the 10th postoperative day. He presented with complaints of cystic swelling at operative site with high-grade fever. Aspiration of swelling was done which showed frank pus, it was sent for culture and sensitivity. Culture report showed Staphylococcus aureus. Intravenous antibiotics were started according to culture sensitivity report and intravenous antibiotic was given for 2 weeks.
Basheer et al.[
Gooch et al.[
Zain et al.[
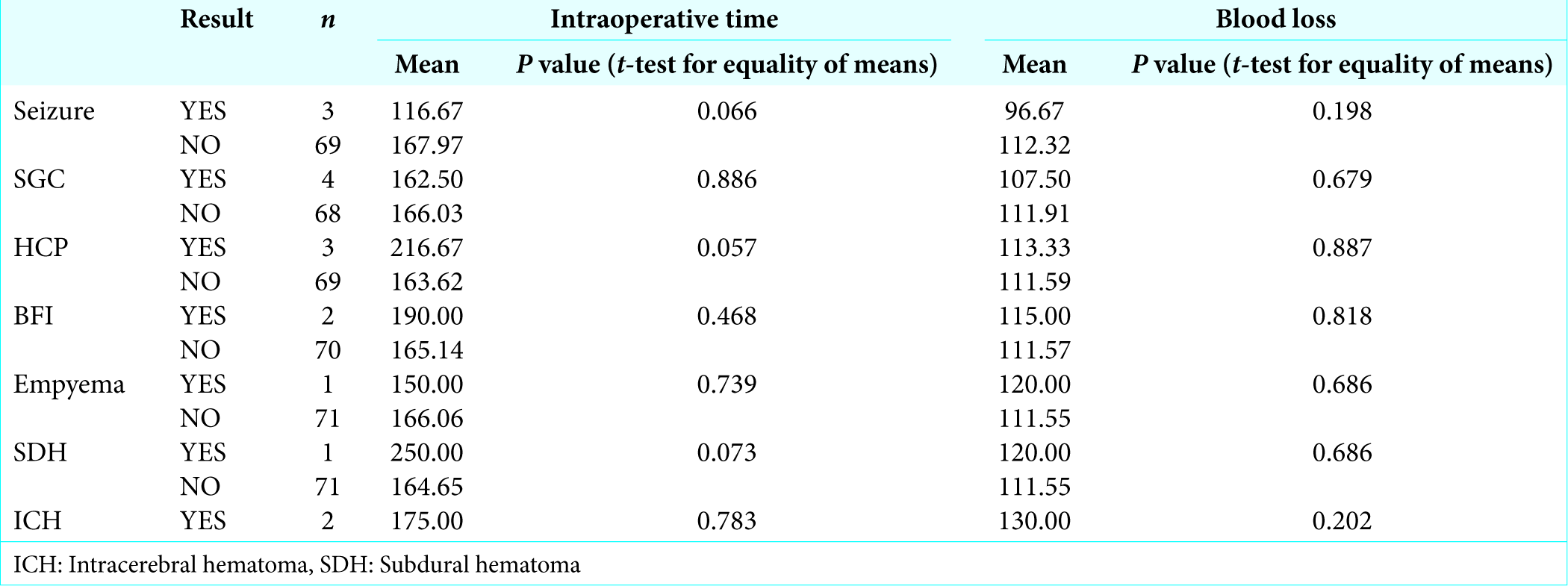
Statistical analyses were done to find out relation of complication with comorbidities such as hypertension, DM, and use of antiplatelet drug. Intraoperative events such as blood loss and operative time also assessed in relation to complications [
Timing of cranioplasty
In our study, we found that the patients who were operated before 84 days (12 weeks) had higher rate of hydrocephalus, seizure, empyema, subdural hematoma, and intracerebral hematoma while patents who were operated after 84 days (12 weeks) had higher rate of bone flap infection and subgaleal collection, but these findings were not statistically significant (P > 0.05).
Our results are slightly similar to a study done by Walcott et al.[
Basheer et al.[
The advantage of timely cranioplasty is easier tissue dissection due to well-maintained plane between dura mater and subgaleal till 4 and 5 months postcraniectomy. It also counters negative postcraniectomy complications such as hydrocephalus and syndrome of trephine.
Complication requiring resurgery
In our study, six patients (8.4%) had to be reoperated due to complications. The most common indication for reoperation was hydrocephalus accounting for three patients. Two patients of bone flap infection and one patient of SDH required reoperation.
Among the patient having hydrocephalus, two patients presented with headache while one patient presented with decrease level of consciousness and elevation of bone flap. All patients were got operated and Codman Hakim programmable shunt was placed in all patients.
Two patients (2.8%) developed bone flap infection both were reoperated and bone flap was removed and cranioplasty was done later on using polymethyl methacrylate cranioplasty.
Our reoperation rate was 8.4% which was significantly lower as compared to the previous study Basheer et al.[
Gooch et al.[
In our series, 70 of 72 patients were having cranioplasty from the bone placed in bone bank.
Still, our complication rate and reoperation rate were much less than other studies. Hence, bone storage in bone bank as compared to abdomen is not a factor for increase in complication rate. However, as per our experience, storing bone in bone bank avoids unnecessary abdominal incision, blood loss and it decreases operating time also.
Neurological outcome
Clinical outcome in terms of GCS and GOSE was compared precranioplasty, 1 month, 3 months, and 6 months postcranioplasty in our series. Both mean values of GCS and GOSE showed a significant improvement at 3 months and 6 months postcranioplasty compared to precranioplasty. These clinical data were corresponding to the studies done by Chibbaro et al.,[
How to improve results for upcoming neurosurgeons
As cranioplasty is an elective and rewarding procedure, here are some effective ways to handle it with best positive results. Patient should be given necessary time to control his hypertension and uncontrolled DM. Blood thinners should be stopped 7 days before operative procedure to correct dysfunctional coagulation parameters. Intraoperative good blood pressure control is also necessary. These measures usually avoid subdural or epidural hematoma. Shortening intraoperative time and diminishing blood loss give better results for patients. Intraoperative use of antibiotic (vancomycin) containing solution for irrigation is the most beneficial method to avoid bone flap infection, abscess, or empyema. If vancomycin is not available, then usage of any broad-spectrum antibiotic having good Gram- positive bacterial coverage will give good results. Meticulous intermittent closure of subgaleal and skin with a negative suction subgaleal drain for 48 hour is also very helpful in avoiding subgaleal collection or infection. After drain removal, crepe bandage application with moderate pressure for 7 days is also helpful in giving good results in my recent cases. Subgaleal and skin closure over the bone flap should be tension free and stretching should be avoided. Tension bound approximation leads to flap ischemia and necrosis. Unhealthy and irregular bony edges should be nibbled, especially along sphenoid ridge to avoid stretching. Intraoperatively, meticulous dissection should be done between subgaleal and dura to avoid dural breach and cerebrospinal fluid leak. Ideally, cranioplasty should be done between 12 and 16 weeks. Otherwise longer time interval between primary surgery and cranioplasty will lead to dense adhesions making it difficult to obtain the right plane. Bone flap should always be autoclaved before keeping it back. In case of infection or suspicion of contamination, antibiotics should be stepped up. Abscess or empyema should be drained and bone flap should be removed as early as possible and replacement should be done with bone cement. Utilizing these methods, we are getting better results in our recent cases.
CONCLUSION
Cranioplasty after decompressive craniectomy is associated with higher complication rate, but good neurological outcome after surgery always outweighs the complications. However, complications rate can be brought down by meticulous timing of cranioplasty in a patient of well- controlled comorbidities and precise surgical techniques. However, storing bone in bone bank is not an additional factor for any postcranioplasty complications which was considered previously.
Recommendation
As cranioplasty is a relatively simple procedure, a relatively high complication rate in literature needs larger studies to find the associations of complications with comorbidity, intraoperative events, and timing of cranioplasty to minimize the risk of complications.
References
1. Basheer N, Gupta D, Mahapatra AK, Gurjar H. Cranioplasty following decompressive craniectomy in traumatic brain injury: Experience at level-I. Indian J Neurotrauma. 2010. 7: 140-4
2. Beauchamp KM, Kashuk J, Moore EE, Bolles G, Rabb C, Seinfeld J. Cranioplasty after postinjury decompressive craniectomy: Is timing of the essence?. J Trauma. 2010. 69: 270-4
3. Chang V, Hartzfeld P, Langlois M, Mahmood A, Seyfried D. Outcomes of cranial repair after craniectomy. J Neurosurg. 2010. 112: 1120-4
4. Chibbaro S, Di Rocco F, Mirone G, Fricia M, Makiese O, Di Emidio P. Decompressive craniectomy and early cranioplasty for the management of severe head injury: A prospective multicenter study on 147 patients. World Neurosurg. 2011. 75: 558-62
5. Chun HJ, Yi HJ. Efficacy and safety of early cranioplasty, at least within 1 month. J Craniofac Surg. 2011. 22: 203-7
6. De Bonis P, Frassanito P, Mangiola A, Nucci CG, Anile C, Pompucci A. Cranial repair: How complicated is filling a “hole”?. J Neurotrauma. 2012. 29: 1071-6
7. Gooch MR, Gin GE, Kenning TJ, German JW. Complications of cranioplasty following decompressive craniectomy: Analysis of 62 cases. Neurosurg Focus. 2009. 26: E9-
8. Grant GA, Jolley M, Ellenbogen RG, Roberts TS, Gruss JR, Loeser JD. Failure of autologous bone-assisted cranioplasty following decompressive craniectomy in children and adolescents. J Neurosurg. 2004. 100: 163-8
9. Kuo JR, Wang CC, Chio CC, Cheng TJ. Neurological improvement after cranioplasty analysis by transcranial doppler ultrasonography. J Clin Neurosci. 2004. 11: 486-9
10. Liang W, Xiaofeng Y, Weiguo L, Gang S, Xuesheng Z, Fei C. Cranioplasty of large cranial defect at an early stage after decompressive craniectomy performed for severe head trauma. J Craniofac Surg. 2007. 18: 526-32
11. Matsuno A, Tanaka H, Iwamuro H, Takanashi S, Miyawaki S, Nakashima M. Analyses of the factors influencing bone graft infection after delayed cranioplasty. Acta Neurochir (Wien). 2006. 148: 535-40
12. Moreira-Gonzalez A, Jackson IT, Miyawaki T, Barakat K, DiNick V. Clinical outcome in cranioplasty: Critical review in long-term follow-up. J Craniofac Surg. 2003. 14: 144-53
13. Petty PG. Cranioplasty: A follow-up study. Med J Aust. 1974. 2: 806-8
14. Sanan A, Haines SJ. Repairing holes in the head: A history of cranioplasty. Neurosurgery. 1997. 40: 588-603
15. Segal DH, Oppenheim JS, Murovic JA. Neurological recovery after cranioplasty. Neurosurgery. 1994. 34: 729-31
16. Sobani ZA, Shamim MS, Zafar SN, Qadeer M, Bilal N, Murtaza SG. Cranioplasty after decompressive craniectomy: An institutional audit and analysis of factors related to complications. Surg Neurol Int. 2011. 2: 123-
17. Suzuki N, Suzuki S, Iwabuchi T. Neurological improvement after cranioplasty. Analysis by dynamic CT scan. Acta Neurochir (Wien). 1993. 122: 49-53
18. Walcott BP, Kwon CS, Sheth SA, Fehnel CR, Koffie RM, Asaad WF. Predictors of cranioplasty complications in stroke and trauma patients. J Neurosurg. 2013. 118: 757-62