- Department of Neurosurgery, Azienda Ospedaliero Universitaria di Sassari, Via Enrico De Nicola 1, 07100 Sassari (SS), Italy
- Department of Neurosurgery, Regina Elena National Cancer Institute, Neurosurgery Department, Via Elio Chianesi 53, 00144, Rome, Italy
Correspondence Address:
Domenico Policicchio
Department of Neurosurgery, Regina Elena National Cancer Institute, Neurosurgery Department, Via Elio Chianesi 53, 00144, Rome, Italy
DOI:10.4103/sni.sni_252_18
Copyright: © 2018 Surgical Neurology International This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.How to cite this article: Domenico Policicchio, Giampiero Muggianu, Giosuè Dipellegrini, Riccardo Boccaletti. Delayed diagnosis of post-traumatic aneurysm of distal anterior cerebral artery. 01-Nov-2018;9:222
How to cite this URL: Domenico Policicchio, Giampiero Muggianu, Giosuè Dipellegrini, Riccardo Boccaletti. Delayed diagnosis of post-traumatic aneurysm of distal anterior cerebral artery. 01-Nov-2018;9:222. Available from: http://surgicalneurologyint.com/surgicalint-articles/9065/
Abstract
Background:Traumatic intracranial aneurysms (TICA) are often associated with poor prognosis and should be diagnosed as soon as possible to prevent delayed intracranial hemorrhage and high rates of morbidity/mortality related to bleeding. Diagnosis requires a high index of suspicion. The goal of treatment is to exclude the aneurysm issue with surgical or endovascular methods.
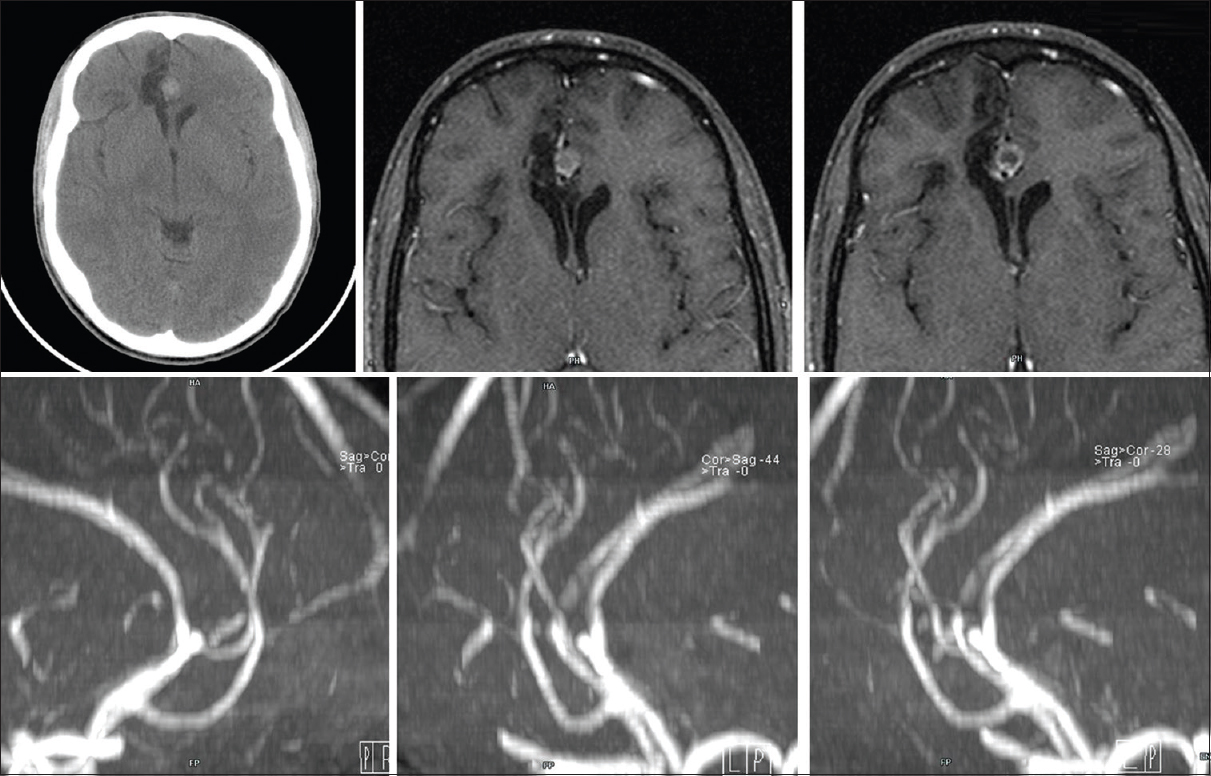
Case Description:We report the case of a 19-year-old boy who suffered a cranio-orbital trauma; 2 weeks after initial trauma he deteriorates with a new intracranial bleeding. Immediate angiography resulted negative. Delayed follow-up by magnetic resonance angiography showed an unruptured aneurysm of anterior cerebral artery that was successfully clipped.
Conclusions:A TICA should be suspected in case of delayed deterioration in head-injured patient, prompt diagnosis and treatment could improve prognosis and reduce morbidity and mortality.
Keywords: Digital subtraction angiography, magnetic resonance angiography, penetrating trauma, post-traumatic aneurysm
INTRODUCTION
Traumatic intracranial aneurysm (TICA) is considered a rare entity, constituting <1% of all cerebral aneurysms.[
CASE DESCRIPTION
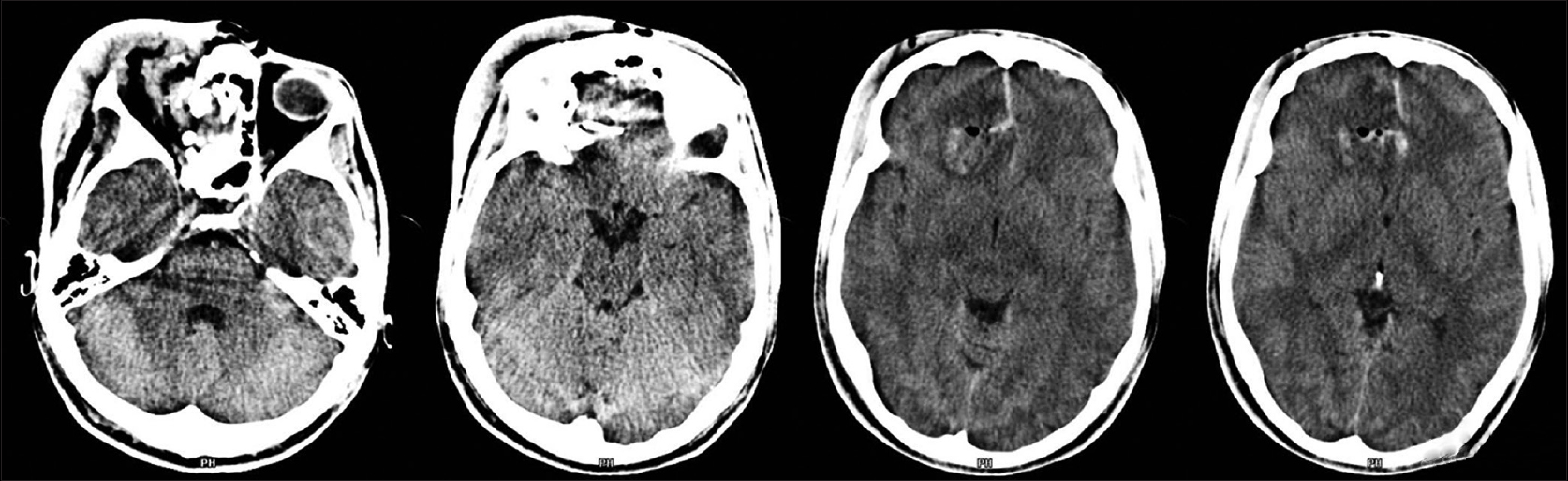
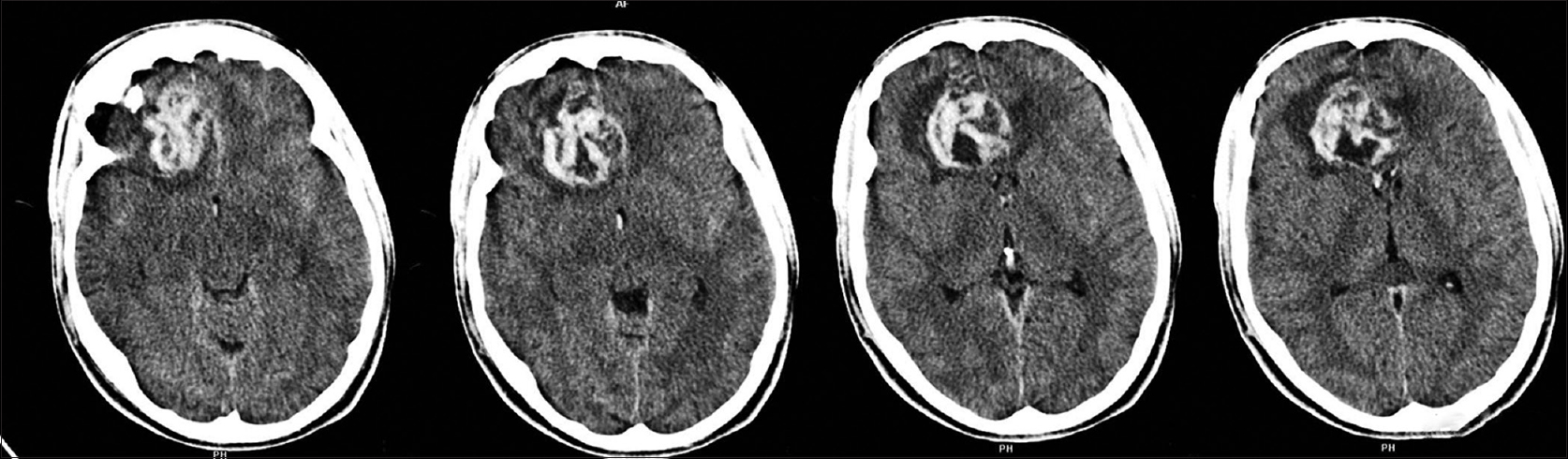
A 19-year-old boy was admitted to our Emergency Department for Cranio-facial Trauma; while he was cutting the grass, a metal object was accidentally hooked to the lawnmower blades and cast at a very high speed towards the boy, hitting him at the right orbit. At admission, the patient was fully alert and orientated, neurological examination showed the Glasgow Coma Scale 15, right blindness, no other neurological deficit, severe swelling of right periorbital tissue, massive conjunctival hemorrhage and swelling. A non-contrast enhanced computed tomography (CT) scan confirmed severe orbital trauma with signs of ruptured globe and injury of the optic nerve; fractures of the medial wall and roof of the right orbit with bone fragments inside the frontal lobe; a small rounded blood effusion in the right paramedian frontal lobe and a slight interhemispheric bleed [
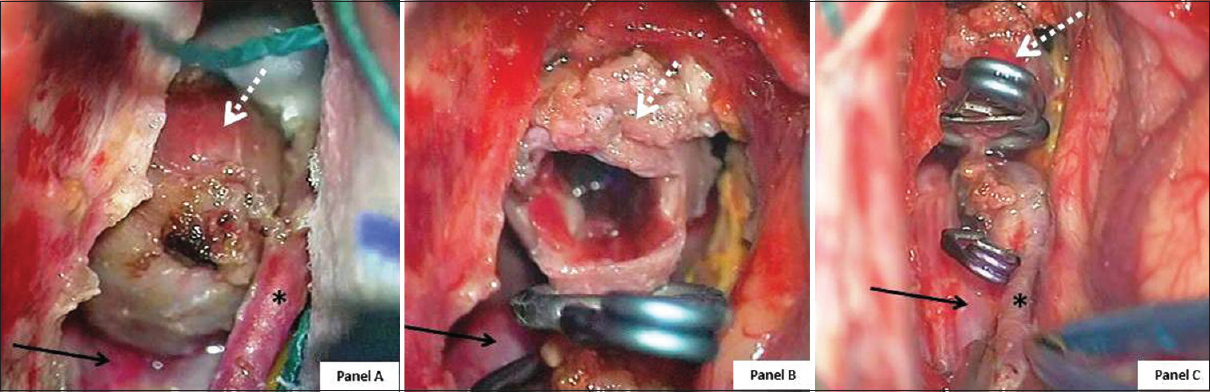
Figure 4
Intraoperative photographs. Panel A: aneurysm completely dissected before clipping. Panel B: aneurysm excluded (using two straight clips) and partially opened. Panel C: enlarged view after clipping, note pericallosal and calloso-marginal artery. (White dotted arrow: aneurysm; black arrow: right pericallosal artery; black asterisk (*): right calloso-marginal artery)
DISCUSSION
TICAs are rare entities, they represent 1% or less of all cerebral aneurysms.[
Although DSA should be considered the gold standard imaging modality in detecting intracranial aneurysm, it is invasive and is associated with radiation exposure and possible allergy to iodinated contrast material; for these reasons several authors investigated the role of MRA as an alternative non-invasive test.[
After established diagnosis of TICA, conservative management is worsened by poor prognosis.[
CONCLUSIONS
Despite their rarity, TICAs are associated with a significant morbidity and mortality rate, as high as 50%.[
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgements
We would like to thank Mrs Lorena Gullà for the English revision.
References
1. Aarabi B. Management of traumatic aneurysms caused by high-velocity missile head wounds. Neurosurg Clin N Am. 1995. 6: 775-97
2. Britz GW, Newell DW, West GA, Winn RH, Winn Richard H.editors. Traumatic cerebral aneurysms secondary to penetrating intracranial injuries. Youmans Neurological Surgery. Philadelphia: Saunders Edition; 2004. p.
3. Buckingham MJ, Crone KR, Ball WS, Tomsick TA, Berger TS, Tew JM. Traumatic intracranial aneurysms in childhood: Two cases and a review of the literature. Neurosurgery. 1988. 22: 398-408
4. Cohen JE, Gomori JM, Segal R, Spivak A, Margolin E, Sviri G. Results of endovascular treatment of traumatic intracranial aneurysms. Neurosurgery. 2008. 63: 476-
5. Du Trevou MD, Van Dellen JR. Penetrating stab wounds to the brain. The timing of angiography in patients presenting with the weapon already removed. Neurosurgery. 1992. 31: 905-11
6. Fleisher AS, Patton JM, Tindall GT. Cerebral aneurysms of traumatic origin. Surg Neurol. 1975. 4: 223-39
7. Handa J, Handa H. Severe epistaxis caused by traumatic aneurysm of cavernous carotid artery. Surg Neurol. 1976. 5: 241-
8. Holmes B, Harbaugh RE. Traumatic intracranial aneurysms: A contemporary review. J Trauma. 1993. 35: 855-60
9. Horowitz MB, Kopitnik TA, Landreneau F, Ramnani DM, Rushing EJ, George E. Multidisciplinary approach to intracranial traumatic aneurysms secondary to shotgun and handgun wounds. Surg Neurol. 1999. 51: 31-42
10. Ida M, Kurisu Y, Yamashita M. MR angiography of ruptured aneurysms in acute subarachnoid hemorrhage. Am J Neuroradiol. 1997. 18: 1025-32
11. Larson PS, Reisner A, Morassutti DJ, Abdulhadi B, Harpring JE. Traumatic intracranial aneurysms. Neurosurg Focus. 2000. 8: e4-
12. Pozzati E, Gaist G, Servadei F. Traumatic aneurysms of the supraclinoid internal carotid artery. J Neurosurg. 1982. 57: 418-22
13. Sailer AM, Wagemans BA, Nelemans PJ, de Graaf R, van Zwam WH. Diagnosing intracranial aneurysms with MR angiography: Systematic review and meta-analysis. Stroke. 2014. 45: 119-26
14. Schuierer G, Huk WJ, Laub G. Magnetic resonance angiography of intracranial aneurysms: Comparison with intra-arterial digital subtraction angiography. Neuroragiology. 1992. 35: 50-4
15. Ventureyra EC, Higgins MJ. Traumatic intracranial aneurysms in childhood and adolescence. Case reports and review of the literature. Childs Nerv Syst. 1994. 10: 361-79
16. Westerlaan HE, van Dijk JM, Jansen-van der Weide MC, de Groot JC, Groen RJ, Mooij JJ. Intracranial aneurysms in patients with subarachnoid hemorrhage: CT angiography as a primary examination tool for diagnosis–systematic review and meta-analysis. Radiology. 2011. 258: 134-45
17. White PM, Wardlaw JM, Easton V. Can noninvasive imaging accurately depict intracranial aneurysms? A systematic review. Radiology. 2000. 217: 361-70