- Section of Neurosurgery, Department of Neurosciences, King Faisal Specialist Hospital and Research Center (Gen. Org) - Jeddah Branch, Jeddah, Saudi Arabia
- Department of Otolaryngology-Head and Neck Surgery, King Abdulaziz University Hospital, King Saud University, Riyadh, Saudi Arabia
- Division of Neurosurgery, Department of Surgery, King Saud University, Riyadh, Saudi Arabia
- Department of Neurosurgery, Stanford University, USA
Correspondence Address:
Abdulrazag M. Ajlan
Division of Neurosurgery, Department of Surgery, King Saud University, Riyadh, Saudi Arabia
Department of Neurosurgery, Stanford University, USA
DOI:10.4103/sni.sni_147_17
Copyright: © 2017 Surgical Neurology International This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Anas M. Bardeesi, Saad Alsaleh, Abdulrazag M. Ajlan. Endoscopic transnasal suprasellar approach for anterior clinoidal meningioma: A case report and review of the literature. 22-Aug-2017;8:194
How to cite this URL: Anas M. Bardeesi, Saad Alsaleh, Abdulrazag M. Ajlan. Endoscopic transnasal suprasellar approach for anterior clinoidal meningioma: A case report and review of the literature. 22-Aug-2017;8:194. Available from: http://surgicalneurologyint.com/surgicalint-articles/endoscopic-transnasal-suprasellar-approach-for-anterior-clinoidal-meningioma-a-case-report-and-review-of-the-literature/
Abstract
Background:Anterior clinoidal meningiomas (ACM) are traditionally approached through transcranial routes. Due to their tendency to extend laterally and their proximity to vital neurovascular structures, the endoscopic transnasal suprasellar approach is still questionable. We present and describe an ACM case that underwent an endoscopic transnasal suprasellar approach, and provide a review of the literature and operative technique.
Case Description:A 56 year-old lady who presented with chronic left-sided decreased vision. Brain imaging revealed a lesion measuring 9 × 10 × 11 mm attached to the left anterior clinoid process (ACP) and extending to the left optic canal. Lesion was compressing the left optic nerve (ON) and abutting the supraclinoid part of the left internal carotid artery (ICA). Utilizing the endoscopic transnasal suprasellar approach, the meningioma was resected and the optic canal was decompressed. Reconstruction was achieved using fascia lata, vomer bone, and nasoseptal flap. A lumbar drain was inserted perioperatively. Patient had no perioperative morbidity and retained vision in the affected eye.
Conclusions:Resection of selected ACMs can be safely achieved utilizing the endoscopic transnasal suprasellar approach. Although the literature lacks long-term outcome comparison between the transnasal and the traditional transcranial approaches, specifically addressing ACMs, this technique is becoming more popular over the last decade. More efforts should be directed towards implementing and reporting the endoscopic transnasal suprasellar approach for meningiomas of the anterior clinoid process.
Keywords: Anterior clinoid process, endoscopic transnasal suprasellar, meningioma
INTRODUCTION
Anterior Clinoidal Meningiomas (ACMs) represent special entity of meningiomas that have been referred to as, or grouped with, suprasellar, parasellar, sphenoid wing, or frontal skull base meningiomas.[
CASE DESCRIPTION
A 56 year-old female who is a known case of hypertension, dyslipidemia, and glaucoma, presented with long-standing decreased vision in her left eye. She had no headaches, no seizures, and no focal motor or sensory symptoms. Her systemic evaluation was irrelevant. On examination, she was conscious, alert, and oriented. Her vitals were within normal ranges. Ophthalmological assessment revealed limited vision to hand motion perception, afferent pupillary defect, superior, inferior, and temporal pallor of the optic disc in the left eye, with fully intact vision and findings in the right eye. She was otherwise neurologically intact.
Brain Magnetic Resonance Imaging (MRI) revealed a small oval-shaped suprasellar extra-axial lesion measuring 9 × 10 × 11 mm attached to the left ACP and part of planum sphenoidale. It was compressing the extracanalicular left ON and extending to the optic canal. The lesion was abutting the supraclinoidal segment of the left ICA.
The patient underwent endoscopic transnasal resection of the extra-axial lesion. After intubation, a lumbar drain was inserted in lateral position. Then, the patient was kept in the supine position and the head was fixed with Mayfield pins (15° right side rotation to the surgeon side, 15° lateral tilt to the right side, minimal flexion). Neuronavigation using Computed Tomography (CT) and Magnetic Resonance Imaging (MRI) with contrast was implemented. Endoscopic partial middle and superior turbinectomy, middle meatal antrostomy, and partial ethmoidectomy were done on the left with exposure of a large Onodi cell on the left. This provided ample exposure of the left ON and orbital apex. Vascularized nasoseptal flaps were raised on both sides. Posterior nasal septectomy and complete removal of the sphenoid sinus face was then done bilaterally. Sphenoid sinus mucosa and bony septae were all removed. The sellar floor, prechiasmatic sulcus and planum sphenoidale were drilled and opened with the aid of Kerrison bone punches and 3 mm burr drill. Dura was coagulated to decrease flow to the tumor. The tumor was identified after opening the dura, and central debulking was done. Resection of the tumor was started medially to laterally using the arachnoid plane. Small residual portion of tumor was left on the ON because of absence of clear separation between the tumor and the nerve. Then, the optic canal was opened using a 3 mm diamond drill to expose the intracanalicular nerve and tumor extension. The intracanalicular tumor was resected from proximal to distal. Defect reconstruction was done in a multilayer fashion with fascia lata, vomer bone, the bilateral nasoseptal flaps and a Merocel® nasal pack [
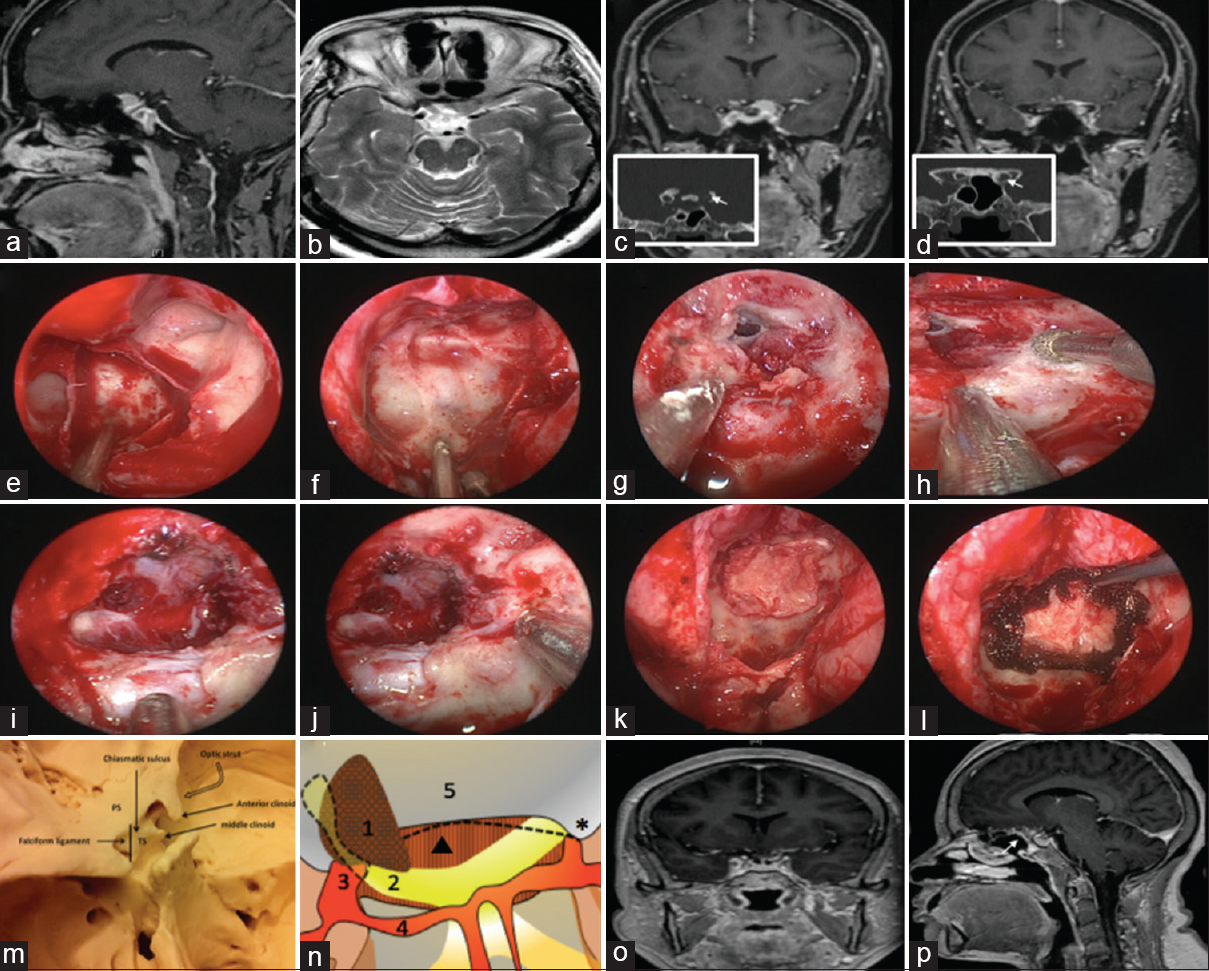
Figure 1
Imaging and approach of ACMs. (a) Preoperative sagittal and (b) axial brain MRI showing the lesion. (c and d) Coronal preoperative MRI with contrast demonstrating the extension of the lesion into the left optic canal. (White arrows) show the enlargement of the left optic canal on coronal CT scan. Intraoperative endoscopic images showing; (e) post drilling of the sphenoid sinus, (f) the sellar floor, (g) dura was opened with partial resection of the tumor and dura over the pituitary gland was kept intact, (h) drilling of the optic canal, (i) tumor resection with residual tumor on the left side covering the optic nerve, (j) Resection of tumor in the optic canal on the lift side. Optic chiasm, right optic nerve and anterior communicating artery complex are observed. (k) Fascia lata, Mepore (gasket seal technique was used) and (l) Surgicel were applied. (m) Superior view of the anterior clinoid and its relation to sellar structures. (n) A superior diagrammatic illustration of the clinoidal lesion compressing the left optic nerve demonstrating; (1) the lesion, (2) the left optic nerve, (3) internal carotid artery, (4) A1 segment, (5) planum sphenoidale, (Dotted line) the falciform ligament, (Triangle) diaphragma sellae and (Star) Right anterior clinoid. (o) Coronal and (p) sagittal MRI with contrast post resection of the lesion with a small part left attached to the left optic nerve. (White arrow) demonstrates the nasoseptal flap in place
Lumbar drain was left in place and the patient had no intraoperative complications. Patient was extubated and transferred to High Dependency Unit, vitally stable within the normal ranges. She was started on Dexamethsone 4 mg intravenously every 6 hours, and Ceftriaxone 1 gram intravenously twice daily, with instructions given to keep the head elevated at 30° and the lumbar drain was set to drain 10 milliliters/hour. The lumbar drain was removed and the patient was discharged home later on the 4th day postoperatively. Histopathological evaluation revealed a meningioma, transitional variant, World Health Organization (WHO) grade 1.
Two weeks after surgery, the patient was re-admitted because of nasal discharge. The nasal discharge was minimal amount of clear fluid with no signs of central nervous system infection. She underwent endoscopic exploration for suspicion of cerebrospinal fluid (CSF) leak. Intraoperatively, no clear site of a leak was identified, but a small mucosal defect in the superior edge of the repair was identified. The edges were freshened and grafted with a free mucosal graft from the nasal floor. The patient was discharged 2 days after the second procedure. The patient was followed in the outpatient clinic for more than 12 months with no evidence of a CSF leak. Visual field and acuity assessment by ophthalmology service confirmed her vision status remained stable with no worsening or improvement in both eyes, with no new complaints or tumor recurrence on follow up brain MRI.
DISCUSSION
The first reported use of a transphenoidal route for tumor resection was in 1907, and that was carried out by Herman Schloffer for a pituitary adenoma.[
The ACP is a bony projection, located medially at the posterior aspect of the lesser sphenoidal wing. From a superior view, it appears as a triangular prominence that directs posteromedially, with anterior and posterior roots attaching its base to the sphenoid bone.[
From an endoscopic view, the sellar floor appears centrally with a clival indentation inferiorly, planum sphenoidale superiorly, two lateral bony projections of the ICAs with two more ON projections superior to them. In between the carotid and optic protuberances on both sides, and depending on the degree of pneumatization of the sphenoid sinus, the lateral opticocarotid recess (OCR) can be identified.[
Comparison of surgical techniques and outcomes
ACMs were classified by Al-Mefty into three groups based on their anatomical relation to the ICA.[
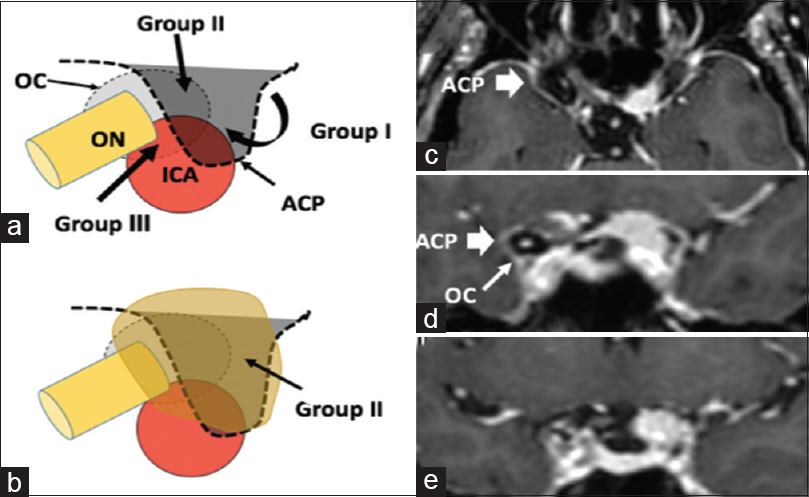
Figure 2
Subgroups of ACMs. (a and b), Schematic drawings illustrating the relation of the three possible groups of ACMs to the surrounding important anatomical structures, where group I are medial to the ACP, the most common group II (Orange shape) arising superiolaterally to the ACP and Group III arising from and extending to the optic canal region. (c) Axial and (d and e) coronal pre-operative brain MRI with contrast showing the right ACP (Thick white arrow), and right optic canal (Thin white arrow) comparing them to the left side with the comparative anatomy invaded by the tumor, in keeping with group II ACM. ACM: Anterior clinoidal meningiomas ACP: Anterior clinoid process, ICA: Internal carotid artery, OC: Optic canal, ON: Optic nerve
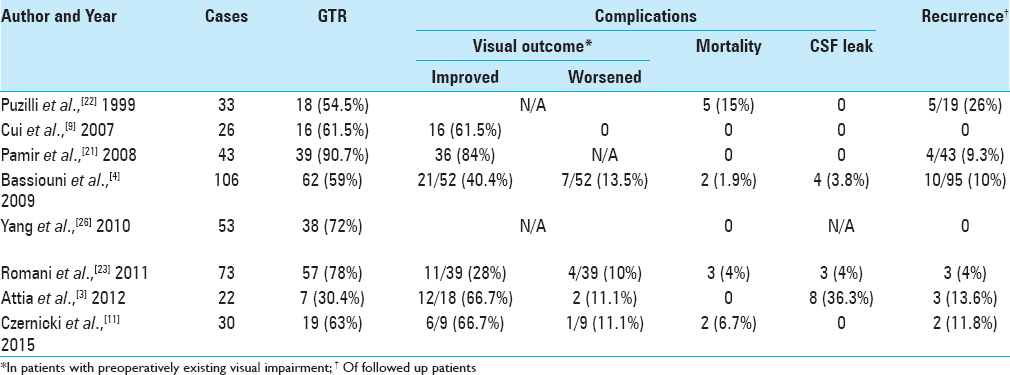
Multiple studies in the literature reported surgical resection of anterior clinoidal meningiomas using traditional transcranial (pterional, extended pterional, frontotemporal, frontoorbital, and frontoorbitozygomatic) approaches, with the pterional approach being mostly implemented.[
Secondary to the close proximity of ACM to the ON, patients tend to present clinically with unilateral visual deterioration,[
Approaching suprasellar lesions through a purely endoscopic transnasal transphenoidal route is becoming an acceptable approach by many of the experts in the field, especially meningiomas. Upon reviewing the literature, we found that majority of studies addressing suprasellar meningiomas, are reporting tuberculum sellae and planum sphenoidale meningiomas specifically, where they were grouped with other anterior skull base lesions. A review article by Ditzel Filho et al.[
Of the reviewed studies approaching anterior skull base meningiomas purely endoscopically, we were able to identify only two studies in which clinoidal meningiomas were included in the series of reported cases. Padhye et al.[
No reported series addressing the pure endoscopic transnasal suprasellar approach for ACMs exclusively, were found in literature. That might be explained mainly by the absence of consensus on the safety of approaching ACMs or suprasellar meningiomas that extend laterally to the ACP. Ottenhausen et al.[
A systematic review was carried out by Clark et al.[
When addressing the advantages of using the endoscopic approach, the visualization it provides with a panoramic view and a light source as close as possible to the lesion, is superior to that provided by the microscope.[
In the last 15 years, endoscopic skull base surgery has raised a growing interest in the neurosurgical community and nowadays is considered a sound alternative to “traditional” skull base surgery in several deep seated lesions, as a rule located in the central skull base. It is not unlikely that increasing experience and refinement of technology will help pure endoscopy to take over most of the space presently still taken by open skull base surgical approaches. However critical be the location, where lesion dissection would require delicate thus dangerous handling of critical neurovascular structure, should be still considered as a rule out of the competence of pure endoscopical procedures. Anterior clinoidectomy, which opens the view to cavernous sinus, medial ICA and optic nerve,[
CONCLUSION
Selected cases of ACMs, despite not being true midline lesions, can still be resected safely through a purely endoscopic transnasal suprasellar approach. Larger series addressing purely endoscopic approach for these types of meningiomas exclusively, separating them from the rest of anterior skull base meninigiomas, is mandated. Proper case selection should also be implied, to achieve the best possible outcomes.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Akture E, Baskaya MK. Microsurgical Anatomy and variations of the Anterior Clinoid Process. Turk Neurosurg. 2014. 24: 484-93
2. Al-Mefty O. Clinoidal meningiomas. J Neurosurg. 1990. 73: 840-9
3. Attia M, Umansky F, Paldor I, Dotan S, Shoshan Y, Spektor S. Giant anterior clinoidal meningiomas: Surgical technique and outcomes: Clinical article. J Neurosurg. 2012. 117: 654-65
4. Bassiouni H, Asgari S, Sandalcioglu IE, Seifert V, Stolke D, Marquardt G. Anterior clinoidal meningiomas: Functional outcome after microsurgical resection in a consecutive series of 106 patients. Clinical article. J Neurosurg. 2009. 111: 1078-90
5. Brunworth J, Padhye V, Bassiouni A, Psaltis A, Floreani S, Robinson S. Update on endoscopic endonasal resection of skull base meningiomas. Int Forum Allergy Rhinol. 2015. 5: 344-352
6. Casiano RR, Numa WA, Falquez AM. Endoscopic resection of esthesioneuroblastoma. Am J Rhinol. 2001. 15: 271-9
7. Chowdhury FH, Haque MR, Goel AH, Kawsar KA. Endoscopic endonasal extended transsphenoidal removal of tuberculum sellae meningioma (TSM): An experience of six cases. Br J Neurosurg. 2012. 26: 692-9
8. Clark AJ, Jahangiri A, Garcia RM, George JR, Sughrue ME, McDermott MW. Endoscopic surgery for tuberculum sellae meningiomas: A systematic review and meta-analysis. Neurosurg Rev. 2013. 36: 349-59
9. Cui H, Wang Y, Yin YH, Fei ZM, Luo QZ, Jiang JY. Surgical management of anterior clinoidal meningiomas: A 26-case report. Surg Neurol. 2007. 68: S6-S10
10. Cushing H, Eisenhardt L. Meningiomas: Their classification. Regional Behavior, Life History and Surgical End Results. Charles C. Thomas, Springfield. 1938. p.
11. Czernicki T, Kunert P, Nowak A, Marchel A. Results of surgical treatment of anterior clinoidal meningiomas–our experiences. Neurol Neurochir Polska. 2015. 49: 29-35
12. Ditzel Filho LF, Prevedello DM, Jamshidi AO, Dolci RL, Kerr EE, Campbell R. Endoscopic Endonasal Approach for Removal of Tuberculum Sellae Meningiomas. Neurosurg Clin N Am. 2015. 26: 349-61
13. Hardy J, Wigser SM. Trans-sphenoidal surgery of pituitary fossa tumors with televised radiofluoroscopic control. J Neurosurg. 1965. 23: 612-9
14. Jho HD. Endoscopic endonasal approach to the optic nerve: A technical note. Minim Invasive Neurosurg. 2001. 44: 190-3
15. Koutourousiou M, Fernandez-Miranda JC, Stefko ST, Wang EW, Snyderman CH, Gardner PA. Endoscopic endonasal surgery for suprasellar meningiomas: Experience with 75 patients: Clinical article. J Neurosurg. 2014. 120: 1326-39
16. Labib MA, Prevedello DM, Fernandez-Miranda JC, Sivakanthan S, Benet A, Morera V. The medial opticocarotid recess: An anatomic study of an endoscopic “key landmark” for the ventral cranial base. Neurosurgery. 2013. 72: ons66-ons76
17. Laufer I, Anand VK, Schwartz TH. Endoscopic, endonasal extended transsphenoidal, transplanum transtuberculum approach for resection of suprasellar lesions. J Neurosurg. 2007. 106: 400-6
18. Lee JH, Sade B.editorsAnterior clinoidal meningiomas. Meningiomas: Springer; 2009. p. 347-354
19. Ottenhausen M, Banu MA, Placantonakis DG, Tsiouris AJ, Khan OH, Anand VK. Endoscopic endonasal resection of suprasellar meningiomas: The importance of case selection and experience in determining extent of resection, visual improvement, and complications. World Neurosurg. 2014. 82: 442-9
20. Padhye V, Naidoo Y, Alexander H, Floreani S, Robinson S, Santoreneos S. Endoscopic endonasal resection of anterior skull base meningiomas. Otolaryngol Head Neck Surg. 2012. 147: 575-82
21. Pamir MN, Belirgen M, Ozduman K, Kilic T, Ozek M. Anterior clinoidal meningiomas: Analysis of 43 consecutive surgically treated cases. Acta Neurochir (Wien). 2008. 150: 625-35
22. Puzzilli F, Ruggeri A, Mastronardi L, Agrillo A, Ferrante L. Anterior clinoidal meningiomas: Report of a series of 33 patients operated on through the pterional approach. Neuro-oncology. 1999. 1: 188-95
23. Romani R, Laakso A, Kangasniemi M, Lehecka M, Hernesniemi J. Lateral supraorbital approach applied to anterior clinoidal meningiomas: Experience with 73 consecutive patients. Neurosurgery. 2011. 68: 1632-47
24. Soni RS, Patel SK, Husain Q, Dahodwala MQ, Eloy JA, Liu JK. From above or below: The controversy and historical evolution of tuberculum sellae meningioma resection from open to endoscopic skull base approaches. J Clin Neurosci. 2014. 21: 559-68
25. Spallone A, Vidal R, Gonzales J. Transcranial approach to pituitary adenomas invading the cavernous sinus: A modification of the classical technique to be used in a low-technology environment. Surg Neurol Int. 2010. 1: 25-
26. Yang YM, Jiang HZ, Sha C, Yuan QG, Xie HW, Wang DM. Microsurgical management of anterior clinoidal meningiomas. Zhonghua Yi Xue Za Zhi. 2010. 90: 1764-6