- Department of Neurosurgery, Azienda Ospedaliero Universitaria di Sassari, Via Enrico De Nicola 1, 07100 Sassari, Italy
- Department of Neurosurgery, Regina Elena National Cancer Institute, Via Elio Chianesi 53, 00144 Rome, Italy
DOI:10.25259/SNI-86-2019
Copyright: © 2019 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Domenico Policicchio, Artan Doda, Giampiero Muggianu, Giosuè Dipellegrini, Riccardo Boccaletti. Ethical and therapeutic dilemmas in glioblastoma management during pregnancy: Two case reports and review of the literature. 26-Mar-2019;10:41
How to cite this URL: Domenico Policicchio, Artan Doda, Giampiero Muggianu, Giosuè Dipellegrini, Riccardo Boccaletti. Ethical and therapeutic dilemmas in glioblastoma management during pregnancy: Two case reports and review of the literature. 26-Mar-2019;10:41. Available from: http://surgicalneurologyint.com/surgicalint-articles/9243/
Abstract
Introduction: There are no guidelines about the management of glioblastoma multiforme (GBM) during pregnancy: treatment of these patients presents therapeutic and ethical challenges.
Case Description: Two patients, respectively, 28 years old at the 14th week of gestation with a thalamic GBM and 38 years old at the 28th week of gestation with fronto-mesial GBM. Patients and their relatives were deeply informed about the natural history of GBM and potential risks and benefits of surgery, radiotherapy (XRT), and chemotherapy (CTX) for both, mother and fetus. The first patient’s will was to preserve her fetus from any related, even minimal, risk of XRT, and CTX until safe delivery despite progression of GBM, accepting only surgery (tumor debulking and shunting of hydrocephalus). The second one asked to deliver the baby as soon as possible (despite the risks of prematurity) to receive the standard treatments of GBM. The two patients survived, respectively, 16 and 46 months after delivery. The first patient’s son is in good clinical conditions; the second one suffered problems linked to prematurity.
Conclusions: Standard treatment of GBM in a pregnant woman could improve the mother’s survival but can expose the fetus to several potential risks. Ethically, relatives should understand that mother has anyway a poor prognosis and, at the same time, fetus prognosis depends on mother’s condition and therapy. It is not possible to warrant absence of risk for both. Considering the absence of guidelines and the relatively poor current data available about management of GBM in a pregnant woman, after a deep explanation of the situation, we think that the will of the mother and her relatives should prevail.
Keywords: Chemotherapy, ethics, glioblastoma, neurosurgery, pregnancy, radiotherapy
INTRODUCTION
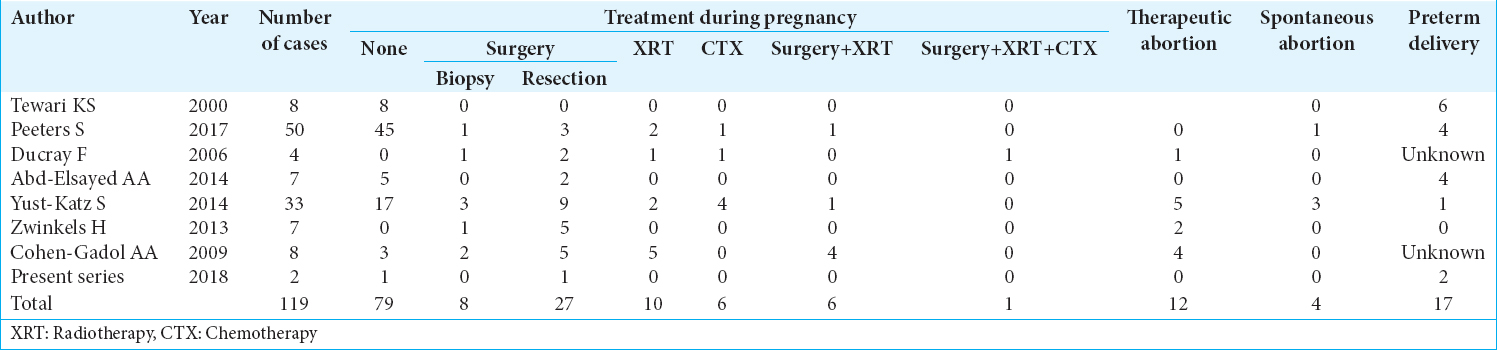
Glioblastoma multiforme (GBM) is the most commonly diagnosed and malignant primary brain tumor. In a pregnant woman, however, it is an unusual and dramatic event because mother, family and physicians must deal with a hard challenge. As far as no current treatment of GBM is curative and there are no clear guidelines about its management in a pregnant woman, the potential benefits in terms of survival to the mother offered by the standard treatment of GBM must be accurately balanced against the potential risks to the fetus. Herein, we present two cases of GBM occurred in pregnant women in different stages of gestational age, review the existing literature and discuss the therapeutic and ethical aspects.
CASE REPORT
Case one
A 28-years-old female at the 14th week of gestation was admitted in April 2016 due to headache, vomiting, and progressive asthenia in the previous 3 weeks. A brain magnetic resonance imaging (MRI) demonstrated a large right thalamic tumor [
Case two
A 38-years-old female, on the 28th week of gestation, presented in November 15, 2014, at the emergency department due to general asthenia associated with a headache, as well as left arm paresis and dysesthesia and a slight left facial deficit. A brain MRI showed a large right frontal tumor [
DISCUSSION
GBM is the most aggressive and malignant primary brain tumor with a poor prognosis. Its standard treatment consists of maximal surgical resection, XRT, and concomitant and adjuvant CTX with TMZ.[
THERAPEUTIC CONSIDERATIONS
Surgery
General considerations for anesthesia in pregnant patients undergoing nonobstetric operations have already been covered in several review articles;[
XRT
The major potential complications of fetal radiation exposure include the death of a developing embryo, teratogenesis, growth retardation, central nervous system (CNS) effects, and induction of malignancy. Developing embryos pass different stages (preimplantation, organogenesis, and fetal growth), with specific sensitivities to the effects of radiation. During the first stage of development, the embryo is sensitive to lethal effects of 0.10 Gy of radiation or less, but it is resistant to teratogenic and growth retarding effects of radiation.[
CTX
TMZ, an alkylating agent used in the treatment of malignant gliomas, is a pregnancy category D medication in USA, UK, and AU and is not advised for use in pregnant women or in those who are contemplating pregnancy. When in some cases, TMZ was used, it was always unintentionally and has been interrupted immediately (unplanned pregnancy in patients already harboring a high-grade glioma).[
Ethical considerations
Glioblastoma is a primitive malignant brain tumor with poor prognosis. Its standard treatment is through surgery, XRT, and CTX.[
Based on our experience and literature data, we proposed a decision-making algorithm, similar to that already published,[
In our first patient, there was a 28-years-old mother at her first pregnancy in the 14th week of the gestational age with a huge right thalamic mass with radiological features of high-grade glioma. The multidisciplinary team which included the intensive care unit consultant, medical oncologist, XRT consultant, high risk obstetrician, and neonatologist as well as psychologist and religious referent, discussed with the mother and her family all the risks and benefits of the pregnancy, the natural history of the disease and the possible outcome as well as the available treatment options. It was decided first to put an EVD and a partial removing of the tumor and to support the mother during her pregnancy without XRT or TMZ to avoid even the minimal risk to the fetus and do elective CS in the week of 24th. After the birth, we perform a gross resection of the remained tumor and begin the XRT and TMZ. We managed to deliver the baby safely at week 24 trying to preserve as much as possible the neurological conditions of the mother. In the second case, the will of the patient and her husband was to deliver the baby as soon as possible and to begin with the standard treatment of GBM. In both cases, we respected their will to avoid any eventual deleterious effects of the fetus in case of beginning XRT and/or TZM during the pregnancy, giving to both the maximal cure.
CONCLUSIONS
When the most malignant primary brain tumor such as GBM, affects a pregnant woman, it becomes a very challenge situation, as far as no current treatment for GBM is curative and there are neither guidelines nor enough evidence about the management of such a dramatic situation in pregnancy. The available literature data suggest that brain surgery during pregnancy can be performed with acceptable risk for mother and fetus; XRT could be administered with increased even though acceptable risks for the fetus; and data are not sufficient to recommend the use of TMZ in pregnancy. Mother has a poor prognosis; fetus’ prognosis depends on mother’s condition and therapy. The chosen approach should involve several professional roles, the patient and familiars should have a deep explanation of the situation, and their will should prevail.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgements
We would like to thank Mrs Lorena Gullà for the English revision.
References
1. Abd-Elsayed AA, Díaz-Gómez J, Barnett GH, Kurz A, Inton-Santos M, Barsoum S. Acase series discussing the anaesthetic management of pregnant patients with brain tumours. F1000Res. 2013. 2: 92-
2. Blumenthal DT, Parreño MG, Batten J, Chamberlain MC. Management of malignant gliomas during pregnancy: A case series. Cancer. 2008. 113: 3349-54
3. Bondy ML, Scheurer ME, Malmer B, Barnholtz-Sloan JS, Davis FG, Il'yasova D. Brain tumor epidemiology: Consensus from the brain tumor epidemiology consortium. Cancer. 2008. 113: 1953-68
4. Brent RL. Saving lives and changing family histories: Appropriate counseling of pregnant women and men and women of reproductive age, concerning the risk of diagnostic radiation exposures during and before pregnancy. Am J Obstet Gynecol. 2009. 200: 4-24
5. Brent R.L. The effects of embryonic and fetal exposure to X-ray, microwaves, and ultrasound. Clin Obstet Gynecol. 1983. 26: 484-510
6. Cohen-Gadol AA, Friedman JA, Friedman JD, Tubbs RS, Munis JR, Meyer FB. Neurosurgical management of intracranial lesions in the pregnant patient: A 36-year institutional experience and review of the literature. J Neurosurg. 2009. 111: 1150-7
7. Ducray F, Colin P, Cartalat-Carel S, Pelissou-Guyotat I, Mahla K, Audra P. Management of malignant gliomas diagnosed during pregnancy. Rev Neurol (Paris). 2006. 162: 322-9
8. Evans AC, Nelson MB, Dhall G. Pregnancy in a patient with a malignant brain tumor taking temozolomide: Case report and review of the literature. J Pediatr Oncol Nurs. 2015. 32: 326-8
9. Grabowski MM, Recinos PF, Nowacki AS, Schroeder JL, Angelov L, Barnett GH. Residual tumor volume versus extent of resection: Predictors of survival after surgery for glioblastoma. J Neurosurg. 2014. 121: 1115-23
10. Haba Y, Twyman N, Thomas SJ, Overton C, Dendy P, Burnet NG. Radiotherapy for glioma during pregnancy: Fetal dose estimates, risk assessment and clinical management. Clin Oncol (R Coll Radiol). 2004. 16: 210-4
11. Jayasekera BA, Bacon AD, Whitfield PC. Management of glioblastoma multiforme in pregnancy. J Neurosurg. 2012. 116: 1187-94
12. Kal HB, Struikmans H. Radiotherapy during pregnancy: Fact and fiction. Lancet Oncol. 2005. 6: 328-33
13. Lynch JC, Gouvêa F, Emmerich JC, Kokinovrachos G, Pereira C, Welling L. Management strategy for brain tumour diagnosed during pregnancy. Br J Neurosurg. 2011. 25: 225-30
14. Mazonakis M, Damilakis J, Theoharopoulos N, Varveris H, Gourtsoyiannis N. Brain radiotherapy during pregnancy: An analysis of conceptus dose using anthropomorphic phantoms. Br J Radiol. 1999. 72: 274-8
15. NíMhuireachtaigh R, O'Gorman DA. Anesthesia in pregnant patients for nonobstetric surgery. J Clin Anesth. 2006. 18: 60-6
16. Nossek E, Ekstein M, Rimon E, Kupferminc MJ, Ram Z. Neurosurgery and pregnancy. Acta Neurochir (Wien). 2011. 153: 1727-35
17. Peeters S, Pagès M, Gauchotte G, Miquel C, Cartalat-Carel S, Guillamo JS. Interactions between glioma and pregnancy: Insight from a 52-case multicenter series. J Neurosurg. 2018. 128: 3-13
18. Sneed PK, Albright NW, Wara WM, Prados MD, Wilson CB. Fetal dose estimates for radiotherapy of brain tumors during pregnancy. Int J Radiat Oncol Biol Phys. 1995. 32: 823-30
19. Stupp R, Hegi ME, Mason WP, Van den Bent MJ, Taphoornm MJ, Janzer RC. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in GBL in a randomized phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009. 10: 459-66
20. Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005. 352: 987-96
21. Tewari KS, Cappuccini F, Asrat T, Flamm BL, Carpenter SE, Disaia PJ. Obstetric emergencies precipitated by malignant brain tumors. Am J Obstet Gynecol. 2000. 182: 1215-21
22. Upadya M, Saneesh PJ. Anaesthesia for non-obstetric surgery during pregnancy. Indian J Anaesth. 2016. 60: 234-41
23. Wu J, Ma YH, Wang TL. Glioma in the third trimester of pregnancy: Two cases and a review of the literature. Oncol Lett. 2013. 5: 943-6
24. Yust-Katz S, de Groot JF, Liu D, Wu J, Yuan Y, Anderson MD. Pregnancy and glial brain tumors. Neuro Oncol. 2014. 16: 1289-94
25. Zwinkels H, Dörr J, Kloet F, Taphoorn MJ, Vecht CJ. Pregnancy in women with gliomas: A case-series and review of the literature. J Neurooncol. 2013. 115: 293-301