- Department of Neurosurgery, Aga Khan University Hospital, Karachi, Pakistan
Correspondence Address:
Asra Tanwir
Department of Neurosurgery, Aga Khan University Hospital, Karachi, Pakistan
DOI:10.4103/sni.sni_242_18
Copyright: © 2018 Surgical Neurology International This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.How to cite this article: Asra Tanwir, Sarmad Bukhari, Muhammad Shahzad Shamim. Frontoethmoidal encephalocele presenting in concert with schizencephaly. 04-Dec-2018;9:246
How to cite this URL: Asra Tanwir, Sarmad Bukhari, Muhammad Shahzad Shamim. Frontoethmoidal encephalocele presenting in concert with schizencephaly. 04-Dec-2018;9:246. Available from: http://surgicalneurologyint.com/surgicalint-articles/9108/
Abstract
Background:Schizencephaly is a rare defect which is identified as clefts that are lined with grey matter extending from the ependyma of the cerebral ventricles to the pia mater. An encephalocele occurs due to failure of neural tube closure resulting in a gap through which cerebrospinal fluid and meninges can bulge into a pouch. There have been rare instances when these two defects have presented simultaneously.
Case Description:We report a case of a 17-year-old child who was brought by his parents with complaint of swelling over his nose and forehead and aggressive behavior since birth. Magnetic resonance imaging findings were consistent with frontoethmoidal meningoencephalocele with schizencephaly. Lumbar drain was inserted and kept in place for 1 week followed by surgical correction of the defect. Our case is interesting because of delayed presentation as it is a rare entity and its association with schizencephaly.
Conclusion:Encephalocele association with schizencephaly is rare.
Keywords: Frontoethmoidal, hypertelorism, meningoencephalocele, schizencephaly
INTRODUCTION
Meningoencephalocele results from failure of rostral neuropore closure during the fourth week of development or primary defect of mesoderm or ectoderm and involves overlying tissues such as meninges and calvarias. The causative factors are genetic, drugs, nutritional, and environmental factors.[
CLINICAL PRESENTATION
A 17-year-old male child was brought by his parents with complaints of swelling over his forehead and nasal bridge since birth. He underwent primary closure of the swelling at the age of 35 days. Postoperatively, he presented with discharge of clear fluid from the site of incision and discharge was resolved with daily dressing. He remained well for 2 months but swelling gradually started to reappear. The size of the swelling has remained unchanged since then and he had not sought further medical care for this swelling. He was delivered full term at a local hospital. There was no significant antenatal history of intrauterine infections or teratogenic drug use.
On examination, we found a well-behaved child with hypertelorism and a fluctuant swelling over his forehead and nasal bridge approximately 6 × 4.8 cm. The swelling had positive transillumination test and positive cough impulse. There were no other associated anomalies. Milestones were up-to-date. He never went to school because of cosmetic deformity, and as per the parents, the child was extremely aggressive. Neurological examination revealed the extraocular movements to be normal and cranial nerves were grossly intact. There was no pronator drift or distal extremity weakness. He had memory impairment and reduced IQ.
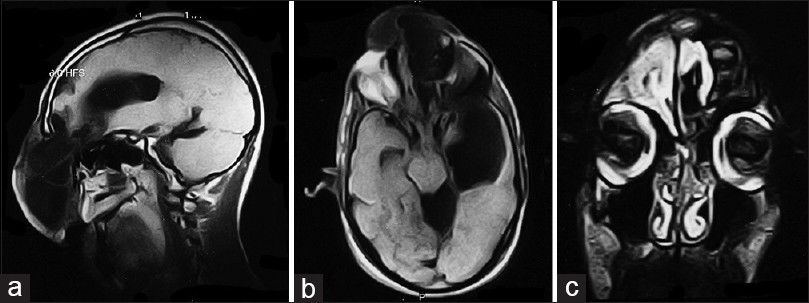
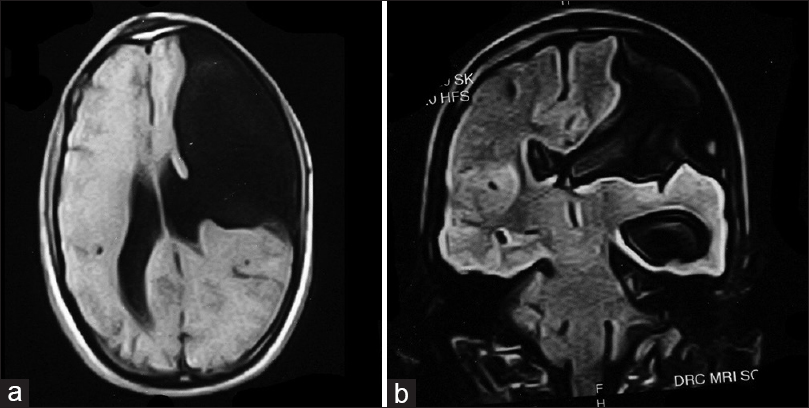
Magnetic resonance imaging (MRI) was consistent with frontoethmoidal meningoencephalocele with schizencephaly [Figures
DISCUSSION
The incidence of encephalocele globally is 1 per 35,000 births, but it is six times more common with 1 in every 6,000 births in South-East Asia.[
The case presented is more interesting due to its association with schizencephaly, a rare birth defect with incidence in the United States estimated at 1.54/100,000 births per year.[
A recently reported study showed relationship between schizencephaly and mutation of the procollagen alpha-1 (IV) (COL4A1) gene.[
Imaging studies help diagnosis of encephaloceles and schizencephaly. T1-weighted images reveal herniated parenchyma as hypotense and hyperintense on T2-weighted images.[
CONCLUSION
Encephalocele association with schizencephaly is rare. While correcting large frontoethmoidal encephalocele, few important points should be considered such as slow decompression of CSF from the lesion and preservation of major veins.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Alexander RC, Patkar AA, Lapointe JS, Flynn SW. Schizencephaly associated with psychosis. J Neurol Neurosurg Psychiatry. 1997. 63: 373-5
2. de Vries LS, Mancini GM. Intracerebral hemorrhage and COL4A1 and COL4A2 mutations, from fetal life into adulthood. Ann Neurol. 2012. 71: 439-41
3. Dobrin N, Mihaela B, Cost B, Tudorache C, Chiriac A, Poeat I. Acquired parietal intradiploic encephalocele. Case report and review of the literature. Romanian Neurosurg. 2011. p. 18-
4. Gould DB, Phalan FC, Breedveld GJ, van Mil SE, Smith RS, Schimenti JC. Mutations in Col4a1 cause perinatal cerebral hemorrhage and porencephaly. Science. 2005. 308: 1167-71
5. Harada T, Uegaki T, Arata K, Tsunetou T, Taniguchi F. Schizencephaly and porencephaly due to fetal intracranial hemorrhage: A report of two cases. Yonago Acta Med. 2018. 60: 241-5
6. Hung PC, Wang HS, Chou ML. Schizencephaly in children: A single medical center retrospective study. Pediatr Neonatol. 2018. 59: 1-8
7. Junaid M, Sobani ZA, Shamim AA, Kazi M, Khan MJ. Nasal encephaloceles presenting at later ages: Experience of Otorhinolaryngology Department at a tertiary care center in Karachi, Pakistan. J Pak Med Assoc. 2012. 62: 74-6
8. Khoshnoodi J, Pedchenko V, Hudson BG. Mammalian collagen IV. Microsc Res Tech. 2008. 71: 357-70
9. Labelle-Dumais C, Dilworth DJ, Harrington EP, de Leau M, Lyons D, Kabaeva Z. COL4A1 mutations cause ocular dysgenesis, neuronal localization defects, and myopathy in mice and Walker-Warburg syndrome in humans. PLoS Genet. 2011. 7: e1002062-
10. Lotfinia I, Mahdkhah A. Intradiploic meningoencephalocele, case report and review of literature. J Clin Exp Neurosci. 2013. 1: 10-
11. Mishra SS, Senapati SB, Das S, Deo RC. Large vertex meningoencephalocele with schizencephaly: An interesting case with neurosurgical challenge. J Pediatr Neurosci. 2014. 9: 136-8
12. Pitkin , Roy M. Folate and neural tube defect. Am J Clin Nutr. 2007. 85: 285S-8S
13. Stopa J, Kucharska-Miąsik I, Dziurzyńska-Białek E, Kostkiewicz A, Solińska A, Zając-Mnich M. Diagnostic imaging and problems of schizencephaly. Pol J Radiol. 2014. 79: 444-9
14. Yoneda Y, Haginoya K, Kato M, Osaka H, Yokochi K, Arai H. Phenotypic spectrum of COL4A1 mutations: Porencephaly to schizencephaly. Ann Neurol. 2013. 73: 48-57