- Department of Neurosurgery, Karolinska University Hospital, Stockholm, Sweden
- Department of Neuroradiology, Karolinska University Hospital, Stockholm, Sweden
- Department of Medical Radiation, Physics and Nuclear Medicine, Karolinska University Hospital, Stockholm, Sweden
- Department of Neuropathology, Karolinska University Hospital, Stockholm, Sweden
Correspondence Address:
Marina Brigui
Department of Neurosurgery, Karolinska University Hospital, Stockholm, Sweden
DOI:10.4103/sni.sni_312_17
Copyright: © 2018 Surgical Neurology International This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Georges Sinclair, Yehya Al-Saffar, Marina Brigui, Heather Martin, Jessica Bystam, Hamza Benmakhlouf, Alia Shamikh, Ernest Dodoo. Gamma knife radiosurgery in the management of endolymphatic sac tumors. 25-Jan-2018;9:18
How to cite this URL: Georges Sinclair, Yehya Al-Saffar, Marina Brigui, Heather Martin, Jessica Bystam, Hamza Benmakhlouf, Alia Shamikh, Ernest Dodoo. Gamma knife radiosurgery in the management of endolymphatic sac tumors. 25-Jan-2018;9:18. Available from: http://surgicalneurologyint.com/surgicalint-articles/gamma-knife-radiosurgery-in-the-management-of-endolymphatic-sac-tumors/
Abstract
Background:Although widely regarded as rare epithelial tumors with a low grade of malignancy, endolymphatic sac tumors (ELST) often lead to disabling petrous bone destruction and significantly impairing symptoms at the time of primary diagnosis and/or recurrence. ELST is not uncommon in von Hippel Lindau (VHL) patients. Although open surgery is regarded as the best treatment option, recurrence remains a challenge, particularly when gross tumor resection (GTR) is deemed unachievable due to topographic conditions. Tumor recurrence successfully treated with fractionated radiotherapy and radiosurgery have been reported in selected cases. We present the case of a patient with recurrent ELST treated with salvage gamma knife radiosurgery (GKRS) adding a review of current literature.
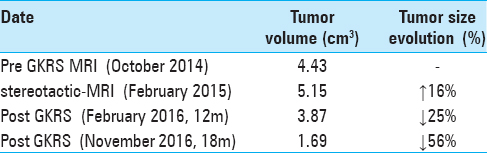
Case Description:A 65-year-old patient underwent GKRS of an unresectable, recurrent ELST. Tumor volumetric analysis showed almost 15% increase in tumor volume in the 4 months between the pre-GKRS magnetic resonance imaging (MRI) and the stereotactic MRI (s-MRI) at treatment. Follow-up MRI at 12 and 20 months showed significant decrease in local tumor volume, decreased contrast enhancement and no perifocal edema. The patient's general and neurological status remains stable to the present day.
Conclusion:In the present case, GKRS was effective in the management of a recurrent ELST over the course of 20 months. Because of ELSTs recurrence potential, long-term follow up is required. The present case as well as previous reports might suggest a possible salvage/adjunctive role of radiosurgery in the management of ELST. Further studies are deemed necessary.
Keywords: Endolymphatic sac tumors, gamma knife radiosurgery, tumor invasiveness, von Hippel Lindau
INTRODUCTION
Endolymphatic sac tumors (ELST) are rare neoplasms derived from the endolymphatic sac of the inner ear. They are slow growing tumors with a low grade of malignancy, but are locally aggressive and destructive.[
This case report describes a sporadic ELST that recurred after three surgical resections and was treated by gamma knife radiosurgery.
CASE PRESENTATION
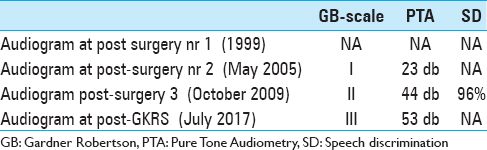
We present the case of a previously healthy, now 67-year-old female patient developing vertigo, nystagmus, wide-based gait, and memory loss throughout July and August 1999 (49 years of age at that time). A Computed Tomography (CT) scan of the brain (August 1999) revealed a 35-mm macrolobulated, extraaxial, brightly contrast enhancing mass in the left posterior fossa with extension along the petrous temporal bone, tentorium cerebelli and sigmoid sinus, with cerebellar edema, compression of the fourth ventricle, supratentorial hydrocephalus as well as erosion of the petrous temporal bone. The tumor was radiologically assessed as a meningioma. Subtotal resection (STR) and ventricular drain placement were performed shortly thereafter (Sept 1999). Audiograms prior and after this surgical intervention are currently not available for review. The patient's condition improved after surgery (pre-operative KPS = 70, post-operative KPS = 90). The initial microscopic evaluation proved complex; after major scrutiny, the pathological report described the tumor as a fibroblastic meningioma.
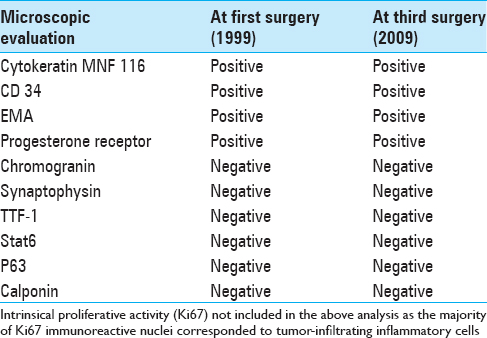
The patient was then lost to follow-up until readmitted six years later (2005) for unilateral progressive hearing loss and pressure like symptoms in her left ear. A clinical examination revealed abnormal bulging of the left tympanic membrane and conductive hearing loss assessed as Gardner-Robertson grade I. A new CT scan and a corresponding MRI (May 2005) showed heterogeneous contrast enhancement suspicious of a local recurrence. The patient underwent a second surgery (August 2005) again assessed as STR. The histopathological examination revealed this time an endolymphatic sac tumor (ELST). Available medical data showed no evidence of von Hippel Lindau (VHL) disease screening prior to both surgeries; to our knowledge, the patient has no known criteria for VHL disease.
Follow-up imaging between 2006 and 2008 showed gradual, but very slow increase in size of a suspected residual/recurrent tumor. A follow-up MRI in February 2009 confirmed a local recurrence in the left sigmoid sinus region and the patient underwent a third surgery (STR) in February 2009. The corresponding pre-operative audiogram is currently not available. The histopathology confirmed an ELST [
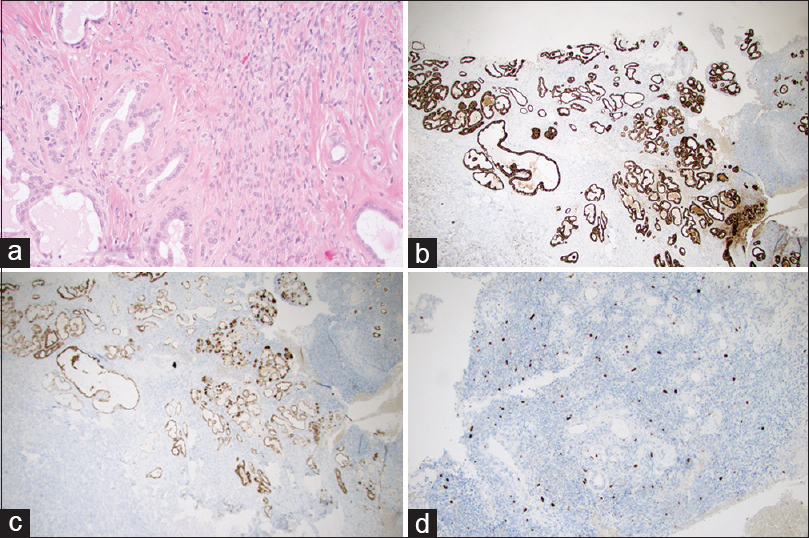
Figure 1
(a–d) The microscopic architecture of ELST at third surgery (Magnification 200×). (a) Hematoxylin and eosin (H and E) staining; (b) CK-MNF immunohistochemical staining positive in glands; (c) EMA immunohistochemical staining positive in glands; (d) Ki67 immunohistochemical staining for proliferative cells
MRI examinations from April 2009 to August 2011 demonstrated no evidence of focal recurrence. Follow-up MRI scans from 2012 to 2013 are currently not available for review. Follow-up MRI in October 2014 showed a local, aggressive recurrence within the lateral limits of the surgical region [
RESULTS
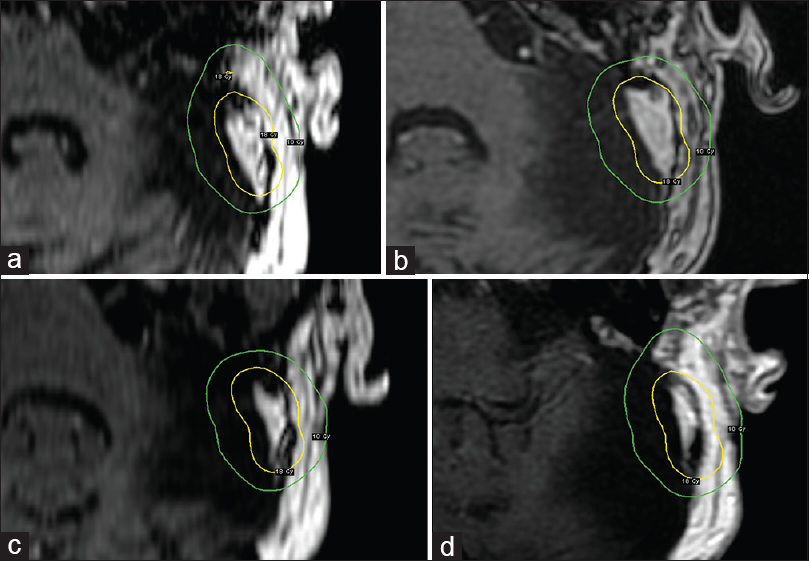
Post-GKRS MRI at 12 months showed a decrease in tumor volume of 25% (3.87cm3) with no evidence of adverse radiation event. A further follow-up MRI at 20 months (November 2016) showed a further decrease of the lesion's volume [Figure
DISCUSSION
ELSTs might present with a complex evolution due to their aggressive erosive growth pattern and recurrence potential. The diagnostics of ELSTs require multidisciplinary assessment; yet, misdiagnoses may still take place as illustrated in our case. We will discuss the traits common to ELSTs in terms of clinical features, diagnostics and available treatments.
Clinical features
ELST was first reported in 1984 after a sac decompression of a presumed unilateral Ménière's disease.[
Microscopic features
According to the WHO, ELSTs are defined as low-grade malignant epithelial tumors of endolymphatic sac origin.[
ELSTs are microscopically composed of papillary fronds and thyroid follicle-like glandular structures. Hemorrhage, calcifications, siderophages, cholesterol clefts, and inflammatory cells are frequently described.[
Neuroimaging
State-of-the-art neuroimaging, including vascular imaging is critical for the diagnosis and management of ELSTs, including MRI, CT with thin slice bone algorithm and digital subtraction angiography (DSA). High-resolution CT often demonstrates a lytic bone lesion in the endolymphatic sac region, effectively delineating the extent of bony erosion which usually involves the retrocochlear posteromedial border of the petrous temporal bone; central spiculated calcifications are also commonly described.[
Treatment
Early GTR is widely regarded as the treatment of choice as it often leads to long disease-free intervals.[
CONCLUSION
Despite being low-grade malignant tumors, ELSTs may present a complex clinical and radiological evolution. Early GTR is widely regarded as first hand treatment but is not always feasible due to local topographic conditions. Cases of recurrence after surgery have been reported and are mainly associated with STR; however, recurrence after GTR may also occur. Repeated resection at the time of recurrence might also prove hazardous due to the previously discussed factors. In these cases, salvage GKRS might have a positive impact in terms of tumor control/tumor progression-free survival; seemingly, good long term results may be achieved prescribing a peripheral dose of 15–18 Gy. Nonetheless, regardless of the treatment modalities chosen and evident positive outcome, we recommend long-term follow up in this group of patients.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient has given her consent for her images and other clinical information to be reported in the journal. The patient understands that name and initial will not be published and due efforts will be made to conceal identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Balasubramaniam S, Deshpande RB, Misra BK. Gamma knife radiosurgery in jugular foramen endolymphatic sac adenocarcinoma. J Clin Neurosci. 2009. 16: 710-1
2. Bambakidis NC, Megerian CA, Ratcheson RA. Differential grading of endolymphatic sac tumor extension by virtue of von Hippel-Lindau disease status. Otol Neurotol. 2004. 25: 773-81
3. Bambakidis NC, Rodrigue T, Megerian CA, Ratcheson RA. Endolymphatic sac tumor metastatic to the spine: Case report. J Neurosurg Spine. 2005. 3: 68-70
4. Carlson ML, Thom JJ, Driscoll CL, Haynes DS, Neff BA, Link MJ. Management of primary and recurrent endolymphatic sac tumors. Otol Neurotol. 2013. 34: 939-43
5. Cheng X, Qin S, Wang X, Li P, Shrestha B, Wang W. Gamma knife treatment of an endolymphatic sac tumor: Unique features of a case and review of the literature. Neurol India. 2011. 59: 608-11
6. Diaz RC, Amjad EH, Sargent EW, LaRouere MJ, Shaia WT. Tumors and Pseudotumors of the Endolymphatic Sac. Skull Base. 2007. 17: 379-93
7. Douglas JG, Goodkin R, Laramore GE. Gamma knife stereotactic radiosurgery for salivary gland neoplasms with base of skull invasion following neutron radiotherapy. Head Neck. 2008. 30: 492-6
8. Dustin MH, David Y, John ML, Elizabeth W, Vikram CP. Myoepithelioma of the Orbital Apex and Middle Cranial Fossa: Case Report and Review of the Literature. Neuroophthalmology. 2014. 38: 14-20
9. Ferreira MA, Feiz EI, Zabramski JM, Spetzler RF, Coons SW, Preul MC. Endolymphatic sac tumor: Unique features of two cases and review of the literature. Acta Neurochir (Wien). 2002. 144: 1047-53
10. Ferri E, Amadori M, Armato E, and Pavon I. A Rare Case of Endolymphatic Sac Tumour: Clinicopathologic Study and Surgical Management. Case Rep Otolaryngol 2014. 2014. p. 376761-
11. Hansen MR, Luxford WM. Surgical outcomes in patients with endolymphatic sac tumors. Laryngoscope. 2004. 114: 1470-4
12. Hassard AD, Boudreau SF, Cron CC. Adenoma of the endolymphatic sac. J Otolaryngol. 1984. 13: 213-6
13. Hagisawa M, Yamasoba T, Ohashi K, Ishida T. A case of middle-ear myoepithelioma. Otolaryngol Head Neck Surg. 2006. 135: 967-8
14. Kamida T, Isono M, Inoue R, Wakabayashi Y, Goda M, Ishii K. Stereotactic radiosurgery for aggressive papillary tumor of the temporal bone: Case report. Surg Neurol. 2002. 58: 124-7
15. Kawaguchi O, Kunieda E, Fujii H, Shigematsu N, Kutsuki S, Itou H. Adenoid cystic carcinoma with hyperostosis after stereotactic radiosurgery. Radiat Med. 2004. 22: 198-200
16. Künzel J, Agaimy A, Hornung J, Lell M, Ganslandt O, Semrau S. Sporadic endolymphatic sac tumor – a diagnostic and therapeutic challenge. Int J Clin Exp Pathol. 2014. 7: 2641-6
17. Nevoux J, Nowak C, Vellin JF, Lepajolec C, Sterkers O, Richard S. Management of endolymphatic sac tumors: Sporadic cases and von Hippel-Lindau disease. Otol Neurotol. 2014. 35: 899-904
18. Patil S, Scheithauer BW, Strom RG, Mafra M, Chicoine MR, Perry A. Malignant meningiomas with epithelial (adenocarcinoma-like) metaplasia: A study of 3 cases. Neurosurgery. 2011. 69: 884-92
19. Poletti AM, Dubey SP, Colombo G, Cugini G, Mazzoni A. Treatment of endolymphatic sac tumour (Papillary adenocarcinoma) of the temporal bone. Rep Pract Oncol Radiother. 2016. 21: 391-4
20. Rodriguez F, Scheithauer BW, Ockner DM, Giannini C. : Solitary fibrous tumor of the cerebellopontine angle with salivary gland heterotopia: A unique presentation. Am J Surg Pathology. 2004. 28: 139-42
21. Sandison A, El Naggar AK.editorsWHO classification of head and neck tumours. IARC press Lyon; 2017. p. 267-8
22. Tay KY, Yu E, Kassel E. Spinal metastasis from endolymphatic sac tumor. AJNR Am J Neuroradiol. 2007. 28: 613-4
23. Tibbs RE, Bowles AP, Raila FA. Should endolymphatic sac tumors be considered part of the von Hippel-Lindau complex?. Neurosurgery. 1997. 40: 848-55
24. Tsuyuguchi N, Ohata K, Goto T, Haque M, Hara M. Intracranial adenoid cystic carcinoma of suprasellar region. Acta Neurochir. 2001. 143: 729-32
25. Virk JS, Randhawa PS, Saeed SR. Endolymphatic sac tumour: Case report and literature review. J Laryngol Otol. 2013. 127: 408-10
26. Yilmaz I, Bolat F, Demirhan B, Aydin V, Ozluoglu LN. Endolymphatic sac papillary tumor: A case report and review. Auris Nasus Larynx. 2008. 35: 276-81
27. Yu SJ, Chen YD, Gao F, Qiu XG, Chang H. Endolymphatic sac papillary tumor: A case report. Chin Med J (Engl). 2011. 124: 3828-9






Jose Mulrain
Posted February 19, 2018, 2:05 am
Very interesting article, with nice illustrations. Thank you!