- Department of Neurosurgery, Fondazione IRCCS Istituto Neurologico C. Besta, Milano, Italy
- Department of Neurological Surgery, Johns Hopkins Medical School, Baltimore, Maryland, USA
Correspondence Address:
Francesco Prada
Department of Neurosurgery, Fondazione IRCCS Istituto Neurologico C. Besta, Milano, Italy
Department of Neurological Surgery, Johns Hopkins Medical School, Baltimore, Maryland, USA
DOI:10.4103/2152-7806.190437
Copyright: © 2016 Surgical Neurology International This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Prada F, Bene MD, DiMeco F. Image guidance in skull base tumor resection: A synergistic approach using intraoperative navigated angiosonography for real-time vessel visualization. Surg Neurol Int 13-Sep-2016;7:82
How to cite this URL: Prada F, Bene MD, DiMeco F. Image guidance in skull base tumor resection: A synergistic approach using intraoperative navigated angiosonography for real-time vessel visualization. Surg Neurol Int 13-Sep-2016;7:82. Available from: http://surgicalneurologyint.com/surgicalint_articles/image-guidance-skull-base-tumor-resection-synergistic-approach-using-intraoperative-navigated-angiosonography-real%e2%80%91time-vessel-visualization/
Sir,
We read with great interest the article by Parviz Dolati et al.,[
The authors report their experience with image-based preoperative vascular and neural element segmentation with three-dimensional (3D) reconstruction for intraoperative guidance in ten patients undergoing surgery for skull base tumor resection. In all cases, the 3D segmented structures were correlated to the correspondent intraoperative findings with the aid of neuronavigation. They conclude that neuronavigation, especially in skull base lesions, is extremely informative for both preoperative planning and intraoperative navigation. Moreover, thanks to the relative immobility of the structures of this area, it is reliable throughout the duration of the surgery.
Preoperative segmentation is noteworthy because it forces to understand the radiological anatomy of the surgical region in order to perform the segmentation, and especially for the less experienced surgeon, it is a great support in understanding the intraoperative relationship between anatomical structures.
The authors rightly point out the innumerable advantages and positive features of neuronavigation in skull-base surgery; the high level of accuracy they have obtained is also extremely interesting.
We completely agree with the authors, however, we would like to underline that neuronavigation, being based on preoperative acquired images, is a dynamic but not a real-time tool and the image dataset is predetermined; thus, it cannot refigure the real intraoperative situation (e.g. it cannot display the re-opening of a sinus that appeared obliterated on the preoperative magnetic resonance imaging (MRI) because it was only compressed by the tumor). Another feature to underline is that navigation displays images that are static, thus it is only an anatomic representation, whereas, especially in skull base lesion, sometimes it could be useful to understand the pathophysiology of the tumor. For instance, it is useful to visualize the principal vascular supplier in a case of meningioma to reduce bleeding. Furthermore, the need of a precise preoperative vessels description leads to acquire a computed tomography angiogram that is obviously a dataset acquired prior to surgery and does not take into account any shifting of the tumor capsule or displaced vessels, also exposing the patient to harmful radiation.
To overcome all these limitations, especially when major vessels are involved, we recommend the use of multimodal navigation with fusion imaging between preoperative MRI and intraoperative contrast enhanced ultrasound (CEUS), obtaining a real-time navigated angiosonography (N-ASG) [Figures
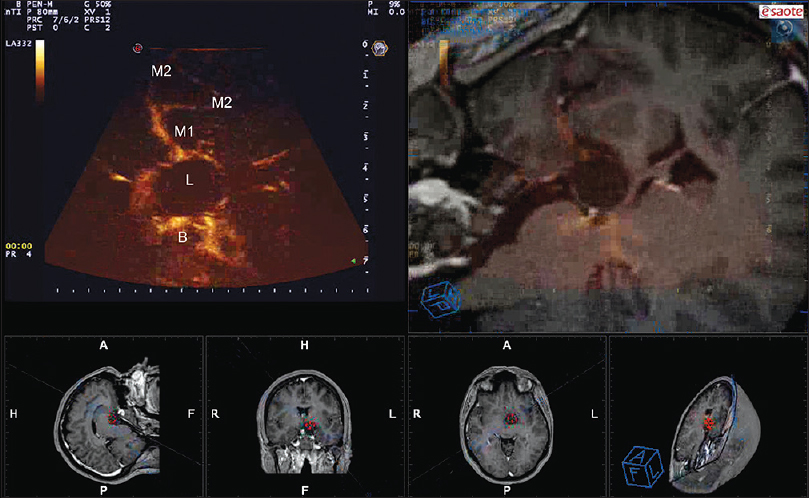
Figure 1
Navigated angio-sonography (N-ASG) screenshot of a craniopharyngioma. In the left upper part of the screen, the ASG image is displayed; on the right, the corresponding preoperative MRI is fused and superimposed with real-time ASG. In the lower portion, the three standard orthogonal planes and the isonation plane are displayed. N-ASG permits to identify the principal vessels surrounding the lesion (L): M1 and M2 portion of the middle cerebral artery and the Basilar tip (B)
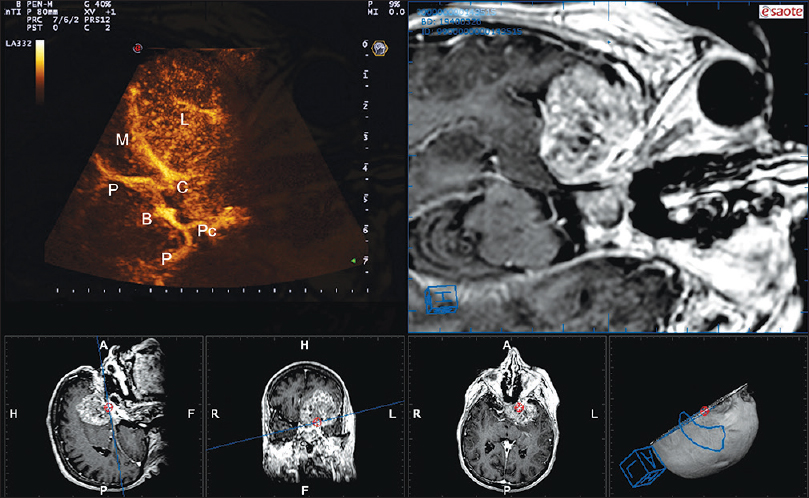
Figure 2
Navigated angio-sonography (N-ASG) screenshot of a sphenoid wing meningioma. In the left upper part of the screen, the ASG image is displayed; on the right, the corresponding preoperative MRI is fused and superimposed with real-time ASG. In the lower portion, the three standard orthogonal planes and the isonation plane are displayed. N-ASG permits to identify the principal vessels surrounding the lesion (L): Middle cerebral artery (M), Basilar tip (B), posterior cerebral artery (P), posterior communicating artery (Pc), internal carotid artery (C)
In conclusion, we believe that a synergistic approach between different imaging modalities is highly desirable; a thorough pre-operative evaluation will obviously lead to a clearer understanding of the surgical anatomy, whereas the real-time imaging modality will provide a continuous feedback in support of neuronavigation.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Dolati P, Gokoglu A, Eichberg D, Zamani A, Golby A, Al-Mefty O. Multimodal navigated skull base tumor resection using image-based vascular and cranial nerve segmentation: A prospective pilot study. Surg Neurol Int. 2015. 6: 172-
2. Prada F, Bene MD, Casali C, Saladino A, Legnani FG, Perin A. Intraoperative Navigated Angiosonography for Skull Base Tumor Surgery. World Neurosurg. 2015. 84: 1699-707
3. Prada F, Del Bene M, Saini M, Ferroli P, DiMeco F. Intraoperative cerebral angiosonography with ultrasound contrast agents: How I do it. Acta Neurochir. 2015. 157: 1025-9
4. Prada F, Perin A, Martegani A, Aiani L, Solbiati L, Lamperti M. Intraoperative contrast-enhanced ultrasound for brain tumor surgery. Neurosurgery. 2014. 74: 542-52