- Department of Anesthesiology, Baylor College of Medicine/Texas Children's Hospital, Houston, TX 77030, USA
- Department of Neurosurgery, Division of Pediatric Neurosurgery, Baylor College of Medicine/Texas Children's Hospital, Houston, TX 77030, USA
Correspondence Address:
Karla E. K. Wyatt
Department of Neurosurgery, Division of Pediatric Neurosurgery, Baylor College of Medicine/Texas Children's Hospital, Houston, TX 77030, USA
DOI:10.4103/sni.sni_151_18
Copyright: © 2018 Surgical Neurology International This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.How to cite this article: Karla E. K. Wyatt, Sandi K. Lam, Nisha Gadgil. Indirect revascularization for moyamoya disease: A pediatric neuroanesthesiology perspective. 02-Nov-2018;9:224
How to cite this URL: Karla E. K. Wyatt, Sandi K. Lam, Nisha Gadgil. Indirect revascularization for moyamoya disease: A pediatric neuroanesthesiology perspective. 02-Nov-2018;9:224. Available from: http://surgicalneurologyint.com/?post_type=surgicalint_articles&p=9063
Keywords: Anesthetic management, anesthetic monitoring, cerebral ischemia, cerebral perfusion pressure, moyamoya, neuroprotective
ILLUSTRATIVE CASE
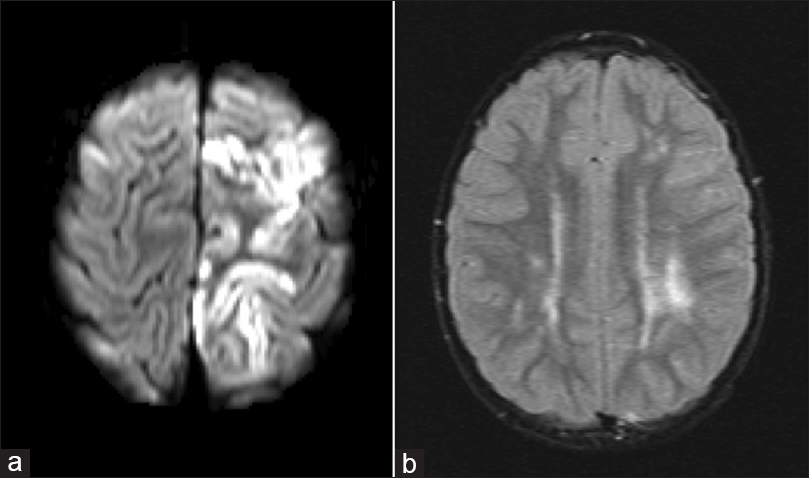
The patient is a 4-year-old male with no significant prior medical history who presented with acute onset of right arm and leg weakness. Neurological examination was significant for 4/5 strength in the right arm and 2/5 strength in the right leg. MRI of the brain was significant for acute cortical infarct of the left frontal lobe and FLAIR signal change of the deep frontal and periventricular white matter consistent remote ischemic insult [Figure
Figure 1
(a) Diffusion weighted magnetic resonance imaging demonstrating diffusion restriction of the left posterior frontal lobe suggesting acute infarct. (b) T2-weighted FLAIR magnetic resonance image demonstrating signal change in the left periventricular subcortical white matter suggestive of remote ischemia
BACKGROUND
Moyamoya disease, a name coined by Suzuki and Takeuchi,[
EPIDEMIOLOGY
Moyamoya disease has a known ethnic and genetic predisposition. The prevalence of moyamoya is highest among Asian countries as compared with the rest of the world. Japan has an estimated incidence of 0.35 to 0.94 per 100,000 people, with a female-to-male ratio of 2:1.[
CLINICAL PRESENTATION, WORKUP, AND DIAGNOSIS
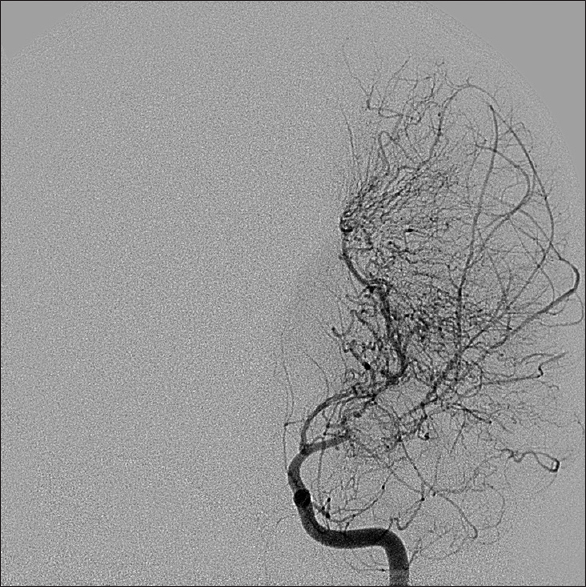
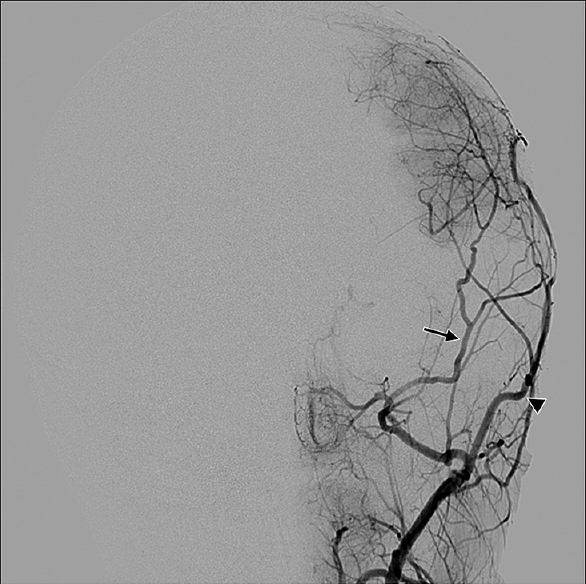
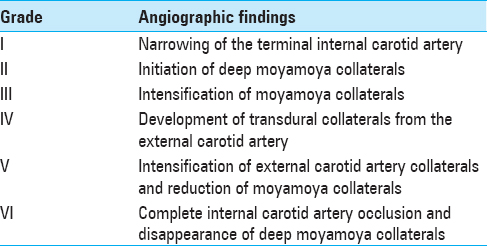
Symptomatology suggestive of moyamoya begins with head computed tomography scan and magnetic resonance imaging (MRI)/magnetic resonance angiography (MRA) to delineate acute versus chronic ischemic infarct. The gold standard for diagnosis of moyamoya disease remains the cerebral angiogram; however, MRA provides a less invasive alternative with concise imaging results.[
Medical treatment is aimed at slowing the progression of vascular changes, reducing the risk of thrombosis, optimizing perfusion in stenotic areas, and seizure control. This is often achieved with a combination of antiplatelet agents, vasodilators, and anticonvulsants. When conservative management strategies are ineffective and/or patients demonstrate evidence of reduced cerebral perfusion, revascularization procedures are performed. Surgical revascularization has been shown to reduce the risk of stroke recurrence.[
ANESTHETIC MANAGEMENT
A multitude of anesthetic techniques and medications have been used for the induction and maintenance of anesthesia in children undergoing interventional and surgical revascularization for moyamoya disease. Pre-eminent to the anesthetic approach is a thorough understanding of the patients' pathology, symptoms, and any underlying comorbid conditions that may further confound anesthetic options.
PREMEDICATION
Anti-anxiolytics, when there are no contraindications, are safe and favorable in helping to blunt the sympathetic and hyperventilatory responses associated with crying, agitation, and parental separation. It is prudent to consider which type of premedication to use so as to avoid sympathetic surge, hypertension, and hypotension.
MONITORING
In addition to the standard American Society of Anesthesiologists[
INDUCTION AND MAINTENANCE OF ANESTHESIA
Both intravenous and inhalational induction of anesthesia is safe and effective in the child with moyamoya. More critical than the technique chosen is the awareness of the potential dangers associated with either. The overarching goals are to maintain cerebral perfusion pressure and thereby cerebral blood flow, avoid hypotension and hypertension, maintain normocarbia, and blunt the sympathetic responses that can increase cerebral oxygen consumption.[
Children, unlike adults, have a high cerebral metabolic oxygen consumption rate (CMRO2) coupled with a high cerebral blood flow (CBF). As such, pediatric patients with moyamoya are at an increased risk for cerebral ischemia once CBF is compromised. Healthy cerebral vasculature will autoregulate changes in mean arterial pressure to maintain CBF; however, in moyamoya this response is diminished for both hypotensive and hypertensive episodes.[
VENTILATION
Arterial carbon dioxide acts to vasodilate the cerebral vasculature (increasing CBF) at high tensions and as a vasoconstrictor (decreasing CBF) at lower tensions. As such, hyperventilating patients with moyamoya can potentiate cerebral ischemia, and this has been shown to cause marked cerebral hypoperfusion through EEG and xenon-based inhalational methods when PaCO2 decreases below 29 mmHg.[
EMERGENCE AND POSTOPERATIVE EVALUATION
The goals of emergence coincide with those of the induction of anesthesia. An immediate postoperative neurological exam is judicious. Extubation of the trachea should occur in a smooth manner so as to avoid the increase in CMRO2 associated with coughing, bucking, agitation, and pain. This can be accomplished with the careful titration of short acting opioids, alpha-2 adrenergic agonists, lidocaine, and/or supplemental short-acting beta-blockers. The postoperative destination will be institution-and surgeon-specific; it is important that the admitting units have the capability to continuously monitor blood pressure, neurological exam, oxygen saturation, hematocrit, and urine output.
CONCLUSION
Moyamoya is a rare but austere disease in the pediatric population. Its presentation and management in children can be complicated by underlying congenital conditions and syndromes. Early diagnosis and treatment are cardinal in preventing neurologic sequelae. Surgical management is effective at reducing symptoms and ischemic recurrence. These children may present to the neuroanesthesiologist for a myriad of procedures. The perioperative anesthetic goals of care are to maintain cerebral perfusion pressure, and thereby cerebral blood flow, avoid hypotension and hypertension, balance normocarbia and normothermia, and blunt the sympathetic responses that can increase cerebral oxygen consumption. Fostered and safe anesthetic management can collaboratively optimize surgical and patient outcomes.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. American Society of Anesthesiologists, Standards and Practice Parameters. Standards for Basic Anesthetic Monitoring; 2015. Last accessed on 2017 Oct 15. Available from: https://www.asahq.org/~/media/Sites/ASAHQ/Files/Public/Resources/standards-guidelines/standards-for-basic-anesthetic-monitoring.pdf.
2. Burke GM, Burke AM, Sherma AK, Hurley MC, Batjer HH, Bendok BR. Moyamoya disease: A summary. Neurosurg Focus. 2009. 26: E11-
3. Guzman R, Lee M, Achrol A, Bell-Stephens T, Kelly M, Do HM. Clinical outcome after 450 revascularization procedures for moyamoya disease. Clinical article. J Neurosurg. 2009. 111: 927-35
4. Lee JK, Williams M, Reyes M, Ahn ES. Cerebrovascular blood pressure autoregulation monitoring and postoperative transient ischemic attack in pediatric moyamoya vasculopathy. Paediatr Anaesth. 2018. 28: 94-102
5. Lee S, Rivkin MJ, Kirton A, deVeber G, Elbers J. Moyamoya disease in children: Results from the international pediatric stroke study. J Child Neurol. 2017. 32: 924-9
6. Ogawa A, Yoshimoto T, Suzuki J, Sakurai Y. Cerebral blood flow in moyamoya disease. Part 1: Correlation with age and regional distribution. Acta Neurochir (Wien). 1990. 105: 30-4
7. Parray T, Martin TW, Siddiqui S. Moyamoya disease: A review of the disease and anesthetic management. J Neurosurg Anesthesiol. 2011. 23: 100-9
8. Scott RM, Smith ER. Moyamoya disease and moyamoya syndrome. N Engl J Med. 2009. 360: 1226-37
9. Soriano SG, Sethna NF, Scott RM. Anesthetic management of children with moyamoya syndrome. Anesth Analg. 1993. 77: 1066-70
10. Suzuki J, Kodama N. Moyamoya disease – A review. Stroke. 1983. 14: 104-9
11. Suzuki J, Takaku A. Cerebrovascular “moyamoya” disease. Disease showing abnormal net-like vessels in base of brain. Arch Neurol. 1969. 20: 288-99
12. Takeuchi K, Shimizu K. Hypoplasia of the bilateral internal carotid arteries. Brain Nerve. 1957. 9: 37-43